Preparation and application of isoflavonoid compound
A technology of isoflavones and compounds, which is applied in the field of new isoflavones and their preparation, can solve the problems of unreported new compounds, etc., and achieve the effect of remarkable ability to scavenge free radicals, low cost, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
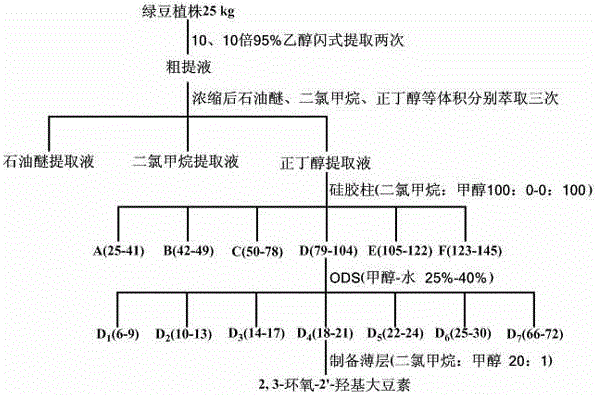
[0045] Embodiment 1, the preparation of 2,3-epoxy-2'-hydroxydatinin
[0046] 1. Plant material: dried stems and branches of leguminous mung bean (Vigna radiate L.).
[0047]2. Separation and preparation method: dry 25 kg of mung bean plant stems, flash extract twice with 10 or 10 times the amount of 95% ethanol, filter, combine the filtrates, recover the ethanol under reduced pressure until there is no alcohol smell, and obtain about 5 L of concentrated solution; Use 5L of petroleum ether, 5L of dichloromethane, and 5L of n-butanol to extract 3 times each, combine the n-butanol extracts, recover n-butanol under reduced pressure, and obtain the mung bean plant extract; disperse the mung bean plant extract with an appropriate amount of methanol, And take an appropriate amount of silica gel to mix the sample; put the mixed sample into a silica gel column, and use dichloromethane-methanol (100:0~0:100) gradient elution to obtain 6 fractions, take fraction 4 [dichloro Methane-meth...
Embodiment 2
[0048] Example 2, In Vitro Antioxidative Activity Test of 2,3-epoxy-2'-hydroxydatinin
[0049] 1. Instruments: U-1900 spectrophotometer (Hitachi), AB135-S electronic balance (Mettler Toledo), microplate reader (BIO-RAD), pH-2TC (0.01 level) precision digital acidity meter (Shanghai Xin Instrument Co., Ltd.).
[0050] 2. Reagents: sodium chloride (NaCl), potassium chloride (KCl), potassium dihydrogen phosphate (KH 2 PO 4 ), dipotassium hydrogen phosphate (K 2 HPO 4 ), potassium persulfate (K 2 S 2 o 8 ), 2,2'-azino-bis-(3-ethylbenzodihydrothiazoline-6-sulfonic acid) diammonium salt (ABTS), ascorbic acid.
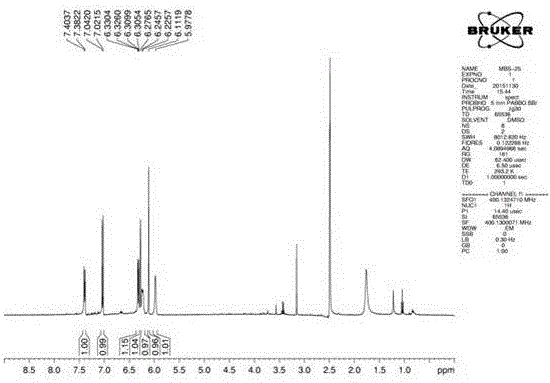
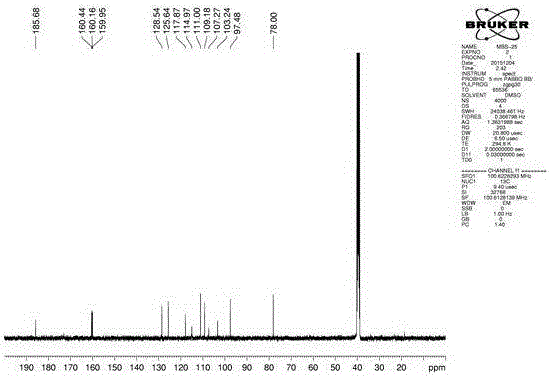
[0051] The 2,3-epoxy-2'-hydroxydaidin used in the experiment was self-made by the inventor, and its chemical structure was confirmed by hydrogen spectrum, carbon spectrum and mass spectrum, and its HPLC purity was above 98%.
[0052] 3. Experimental method: Prepare 7mmol / L ABTS solution and 2.45mmol / L potassium persulfate solution with phosphate buffered saline soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com