Semicarbazide derivatization reagent and rapid detection card thereof
A technology of derivatization reagent and semicarbazide, which is applied in the field of semicarbazide derivatization reagent and rapid detection card, can solve the problems of derivative extraction, destruction of antibody activity, long detection time, etc., and achieve simplified detection steps, simplified separation and purification steps , The effect of reducing the detection time and operation error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
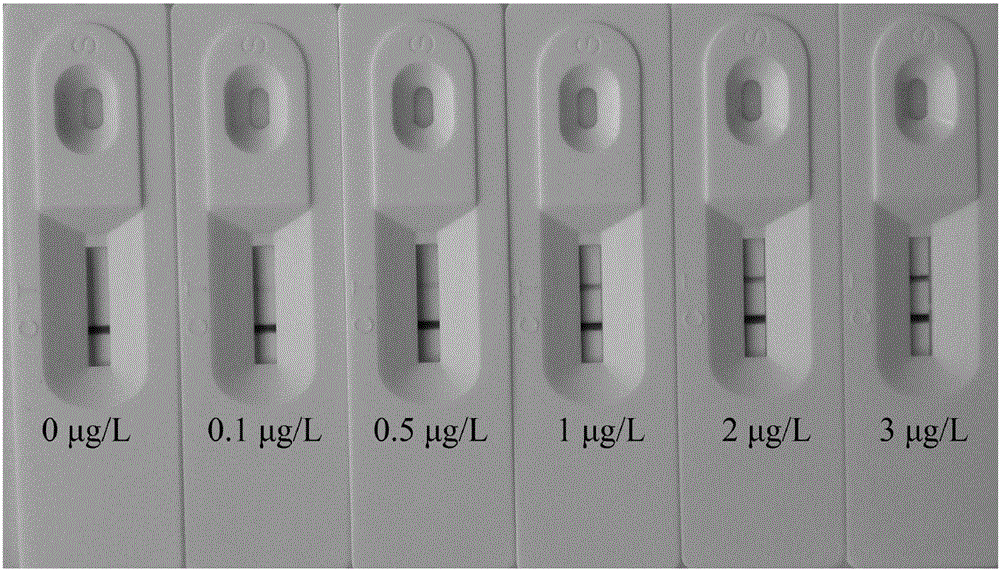
Image
Examples
Embodiment 1
[0023] Take ractopamine-modified p-aldehyde benzoic acid as an example for illustration:
[0024] (1) Preparation of magnetic beads
[0025] Pipette 0.5mL (10mg) of carboxylated magnetic beads into a 15mL centrifuge tube and place on a magnetic separation rack until the supernatant becomes completely transparent, then carefully remove the supernatant with a pipette. Add 5mL of MES buffer, mix well and wash. Place the centrifuge tubes on the magnetic separation rack until the supernatant becomes clear, then carefully remove the supernatant with a pipette. Repeat wash three times. After the last wash, resuspend the magnetic beads in 5 mL of MES buffer. Remove EDAC from refrigerator to room temperature for 30 minutes. Weigh 16 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDAC) into a centrifuge tube containing magnetic beads, shake vigorously. Activate the reaction by placing the tube on a rotator for 30 minutes at room temperature. During the reaction...
Embodiment 2
[0035] Take digoxin-modified p-aldehyde benzoic acid as an example to illustrate:
[0036] (1) Preparation of magnetic beads
[0037] Pipette 0.5mL (10mg) of carboxylated magnetic beads into a 15mL centrifuge tube and place on a magnetic separation rack until the supernatant becomes completely transparent, then carefully remove the supernatant with a pipette. Add 5mL of MES buffer, mix well and wash. Place the centrifuge tubes on the magnetic separation rack until the supernatant becomes clear, then carefully remove the supernatant with a pipette. Repeat wash three times. After the last wash, resuspend the magnetic beads in 5 mL of MES buffer. Remove the EDAC from refrigeration to room temperature for 30 minutes. Weigh 16mg of EDAC and add it into the centrifuge tube containing magnetic beads, shake vigorously. Place the centrifuge tube on a rotary mixer to activate the reaction for 30 minutes at room temperature. During the reaction, be careful not to let the magnetic b...
Embodiment 3
[0047] Take p-aldehyde benzoic acid modified by cyanine dye 5 as an example:
[0048] (1) Preparation of magnetic beads
[0049] Pipette 0.5mL (10mg) of carboxylated magnetic beads into a 15mL centrifuge tube and place on a magnetic separation rack until the supernatant becomes completely transparent, then carefully remove the supernatant with a pipette. Add 5mL of MES buffer, mix well and wash. Place the centrifuge tubes on the magnetic separation rack until the supernatant becomes clear, then carefully remove the supernatant with a pipette. Repeat wash three times. After the last wash, resuspend the magnetic beads in 5 mL of MES buffer. Remove the EDAC from refrigeration to room temperature for 30 minutes. Weigh 16mg of EDAC and add it into the centrifuge tube containing magnetic beads, shake vigorously. Place the centrifuge tube on a rotary mixer to activate the reaction for 30 minutes at room temperature. During the reaction, be careful not to let the magnetic bead p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com