Organic transistor, compound, organic semiconductor material for non-light-emitting organic semiconductor device, material for organic transistor, coating liquid for non-light-emitting organic semiconductor device, method for manufacturing organic transistor, method for manufacturing organic semiconductor film, organic semiconductor film for non-light-emitting organic semiconductor device, and method for synthesizing organic semiconductor material
A technology of organic transistors and semiconductors, applied in semiconductor/solid-state device manufacturing, semiconductor devices, chemical instruments and methods, etc., can solve problems such as evaluation of characteristics of organic transistors without mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and comparative example 1~5
[0561]
[0562] Compound 1 of the present invention was synthesized according to the following route.
[0563] [chemical 14]
[0564] Synthesis of Intermediate 1
[0565]
[0566] [chemical 15]
[0567] Synthesis of Intermediate 2
[0568]
[0569] [chemical 16]
[0570] Synthesis of compound 1
[0571]
[0572] (Synthesis of Intermediate 1)
[0573] Add 23.1ml of tetrahydrofuran to 3.93ml of tetramethylpiperidine (TMP), stir at -78°C, add 13.8ml of n-butyllithium (1.6M hexane solution), raise the temperature to 0°C and stir for 1 hour, prepare the lithium reagent.
[0574] Add 100ml of tetrahydrofuran to 2.969g (10mmol) of thieno[3,2-f:4,5-f']bis[1]benzothiophene, stir at -78°C, and drop at -78°C using a cannula Add the above lithium reagent. The reaction liquid was cooled to -98 degreeC after 2 hours, and the solution which dissolved 9.76 g (30 mmol) of dibromodichloroethanes in 30 ml of tetrahydrofuran was dripped using the cannula. Then, the reaction solu...
Embodiment 13~24 and comparative example 6~8
[0762]
[0763] In Examples 13 to 24 and Comparative Examples 6 to 8, bottom gate-bottom contact type organic transistor devices were produced. The details are as follows.
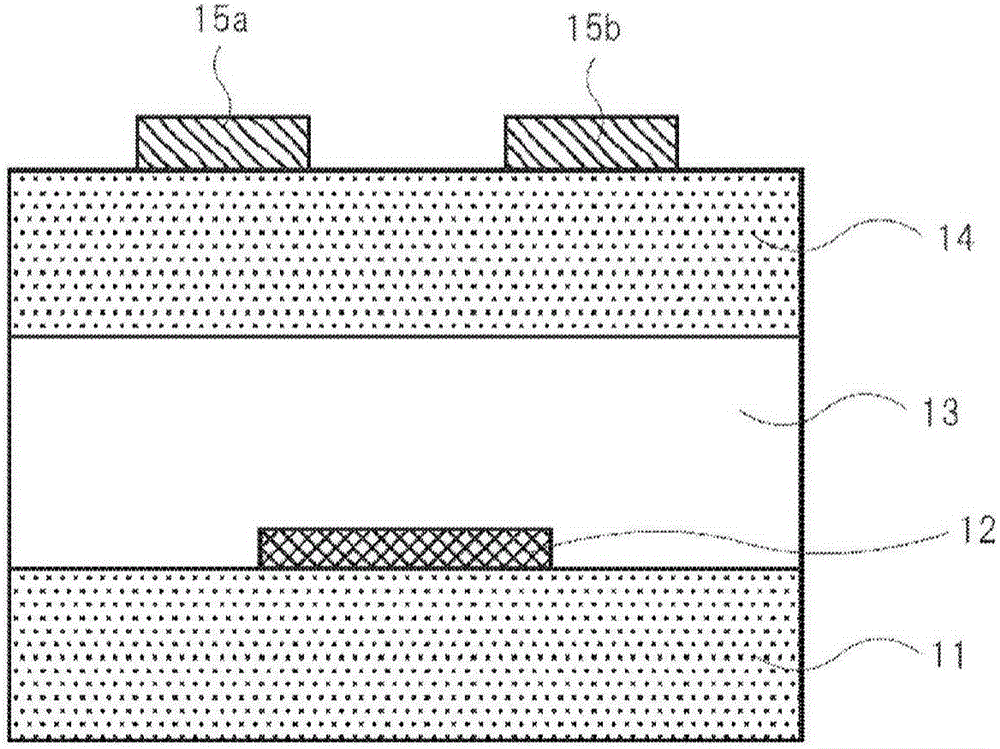
[0764] A 0.1% by mass anisole solution of Compound 1 was heated to 100° C., and the resulting solution was cast in a nitrogen atmosphere on a substrate for measuring FET characteristics heated to 90° C. to obtain a non-luminescent organic transistor element 2 . As the substrate for measuring FET characteristics, chromium / gold (gate width W = 100 mm, gate length L = 100 μm) arranged in a comb-shaped arrangement as the source and drain, and SiO as the insulating film were used. 2 (a silicon substrate with a bottom gate-bottom contact structure with a film thickness of 200nm). The obtained device 13 was used as the organic transistor device of Example 13.
[0765] Devices 14 to 24 and Comparative Devices 6 to 8 were produced in the same manner as in Device 13 except that any one of Compounds 2 to 12 or Co...
Embodiment 25~36 and comparative example 9~11
[0771]
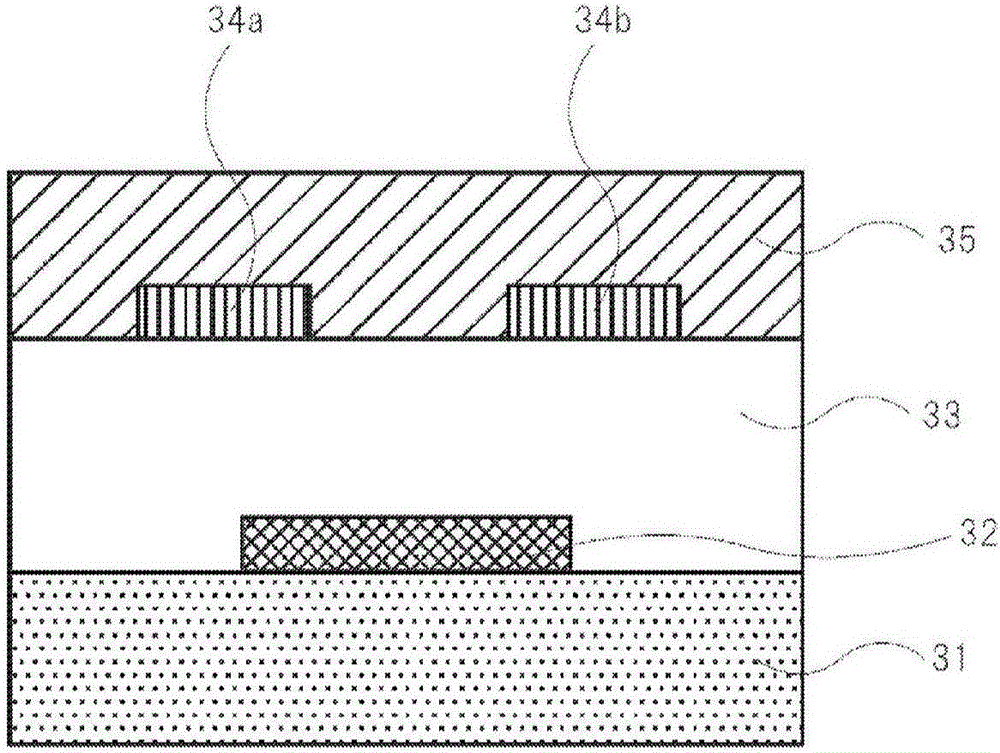
[0772] Bottom gate-bottom contact was produced in the same manner as in Example 13, except that in Example 13, a material containing Compound 1 and poly-α-methylstyrene in a mass ratio of 1:1 (Material 1') was used instead of Compound 1. type element 25. The obtained device 25 was used as the organic transistor device of Example 25.
[0773] Devices 26 to 36 and Comparative Devices 9 to 11 were produced in the same manner as in Device 25 except that any one of Compounds 2 to 12 or Comparative Compounds 1, 2, or 5 was used instead of Compound 1 in the preparation of Device 25 . The obtained elements 26 to 36 and comparative elements 9 to 11 were used as organic transistor elements of Examples 26 to 36 and Comparative Examples 9 to 11. It should be noted that materials containing compounds 2 to 12 and poly-α-methylstyrene at a mass ratio of 1:1 are referred to as materials 2' to 12', respectively.
[0774]
[0775] The FET characteristics of the organic transistor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com