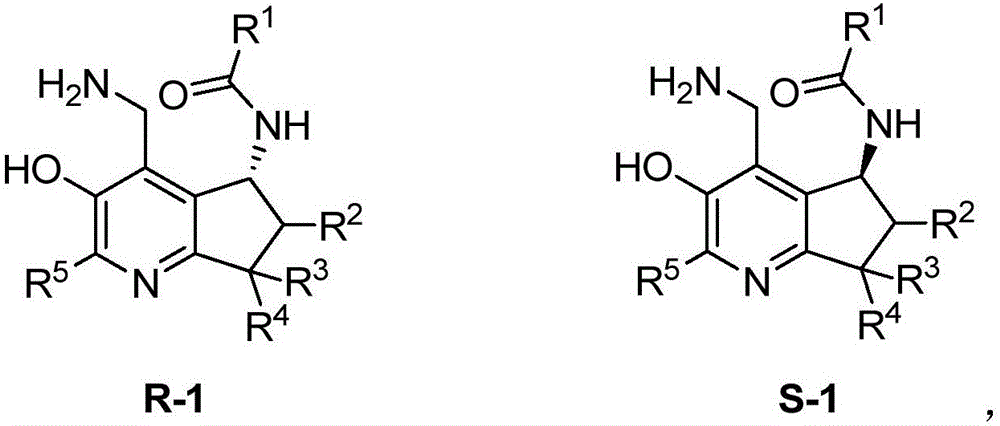
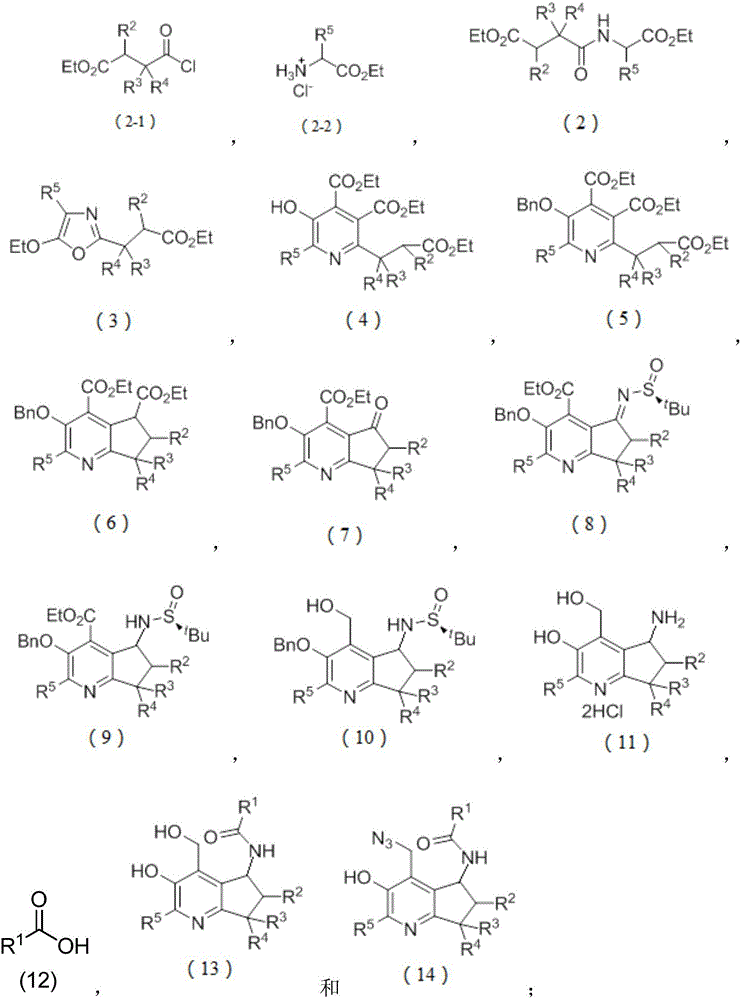
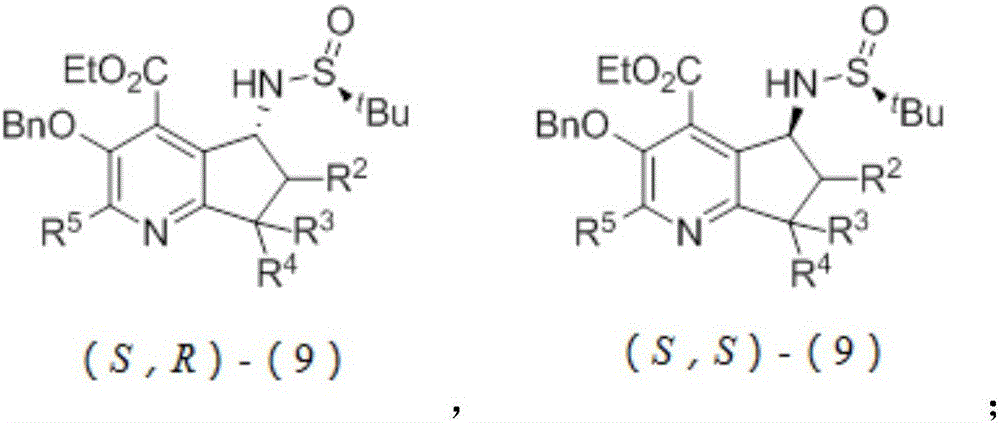
Chiral pyridoxamine catalyst as well as synthesis method and application thereof
A catalyst, pyridoxamine technology, applied in the direction of organic chemistry methods, chemical instruments and methods, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Intermediate 2a (R 2 =R 3 =R 4 =R 5 =H) Synthesis.
[0079]
[0080] Weigh glycine ethyl ester hydrochloride (100.0g, 716.4mmol) in 1000mL DCM (2000mL reaction bottle), add triethylamine (145.2g, 1434.8mmol) under ice bath, after stirring evenly, the constant pressure dropping funnel slowly drops Add succinic acid monoethyl chloride (100 mL, 716.3 mmol), drop it over 30 minutes, and return to room temperature naturally under continuous stirring. TLC tracking monitoring, the reaction is complete after 4h, add 300mL water for extraction, continue to add saturated NaHCO to the organic phase 3 (300mL x2) extraction, the organic phase was dried with anhydrous Na2SO4 for 6h, filtered, and the filtrate was spin-dried to obtain intermediate 2a (white solid, 170.00g, yield 97%). White solid; m.p.72-73℃; IR(KBr)3321,1748,1734,1655,1552,1212cm -1 ; 1 H NMR (600MHz, CDCl 3)δ6.29(s,1H),4.19(q,J=7.2Hz,2H),4.12(q,J=7.2Hz,2H),4.00(d,J=4.8Hz,2H),2.65(t, J=6.6Hz, 2...
Embodiment 1-1
[0082] Example 1-1: except that the molar ratio of glycine ethyl ester hydrochloride, triethylamine and succinic acid monoethyl chloride is 1:1:6, the reaction temperature is -20°C, and the reaction time is 24h, All the other are the same as in Example 1.
Embodiment 1-2
[0083] Example 1-2: Except that the molar ratio of glycine ethyl ester hydrochloride, triethylamine and succinic acid monoethyl chloride is 1:6:3, the reaction temperature is 50°C, and the reaction time is 1h, the rest All the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com