Time-resolved fluorescence immunochromatographic reagent for rapid quantitative simultaneous detection of cTnI, CKMB, Myo and preparation method
A time-resolved fluorescence and immunochromatography technology, applied in the field of clinical medical diagnosis, can solve the problems of background signal interference, inability to meet the sensitivity, multi-sample size, etc., to improve the detection precision and accuracy, reduce the background signal value, eliminate the The effect of sample interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of cTnI, CKMB, Myo time-resolved fluorescent immunochromatographic test strips:
[0062] (1) Preparation of detection line (T1, T2, T3) solution: dilute cTnI monoclonal antibody and polyclonal antibody, CKMB polyclonal antibody, and Myo monoclonal antibody to 1-2 mg with 10-fold, 50 mM citrate solution. / ml;
[0063] (2) Preparation of the quality control line (C) solution: dilute the rabbit IgG antibody to 1-2 mg / ml with 10-fold, 50 mM citrate solution;
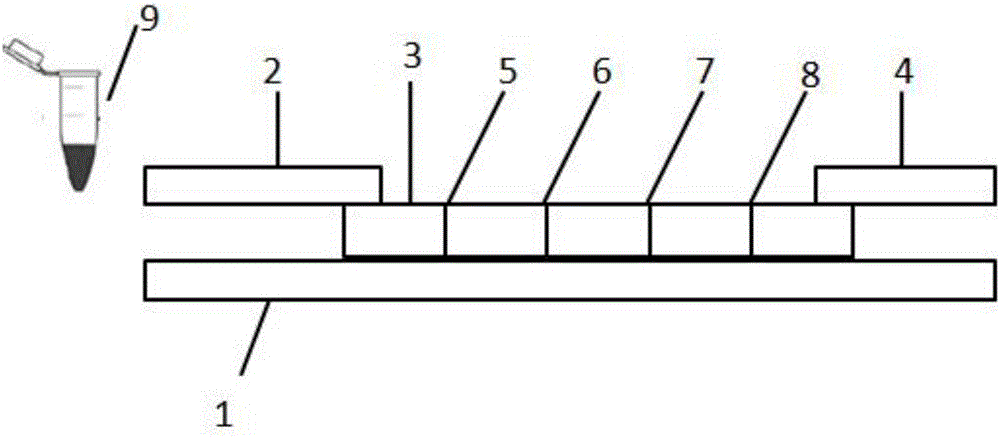
[0064] On the bottom plate (1) with adhesive backing, adopt the overlapping method, first paste the nitrocellulose membrane (3), and then paste the Fusion5 membrane (2) and the absorbent paper ( 4). On the nitrocellulose membrane (3) and close to the Fusion5 membrane (2), draw T1 (Myo capture line (5)), T2 (cTnI capture line (6)), T3 (CKMB capture line (7)), respectively, Draw a C (rabbit IgG) line near one end of the absorbent paper (4), and the distances between T1, T2, T3, and C are all 3mm.
[0065...
Embodiment 2
[0068] Detection of cTnI, CKMB, Myo time-resolved fluorescent immunochromatographic test strips
[0069] (1) Preparation of cTnI, CKMB, Myo, goat anti-rabbit time-resolved fluorescent microspheres
[0070] Dissolve time-resolved fluorescent microspheres (particle size about 200nm) in 100mM MES buffer (pH 6.0), add activator (NHS, 20mg / ml; EDC, 20mg / ml) to activate for 15 minutes; wash, centrifuge, Reconstitute the time-resolved fluorescent microspheres with 60mM boric acid buffer (pH 8.5), then add troponin I monoclonal antibody 2 to react for 2 hours (the mass ratio of microspheres to antibodies is 10mg:0.5mg); the reaction is completed Then add blocking agent (BSA, 100mg / ml) to block for 2 hours; after blocking, wash, centrifuge, redissolve with 60mM boric acid buffer (pH 8.5) and blocking agent (BSA, 100mg / ml) again, sonicate, make The microspheres are evenly dispersed in the buffer, and stored at 2-8°C in the dark.
[0071] The preparation process of fluorescent microsph...
Embodiment 3
[0084] Precision testing
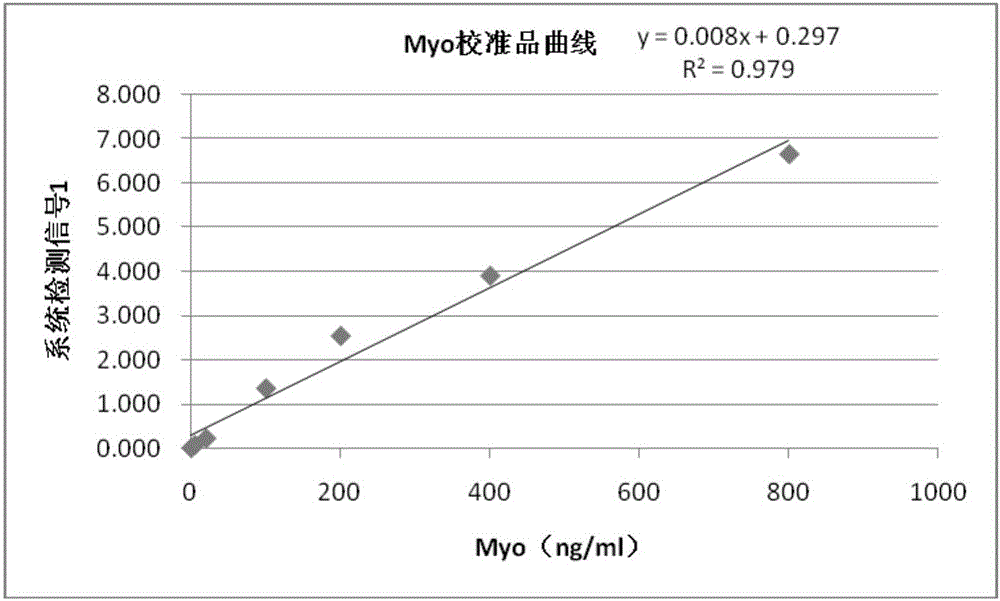
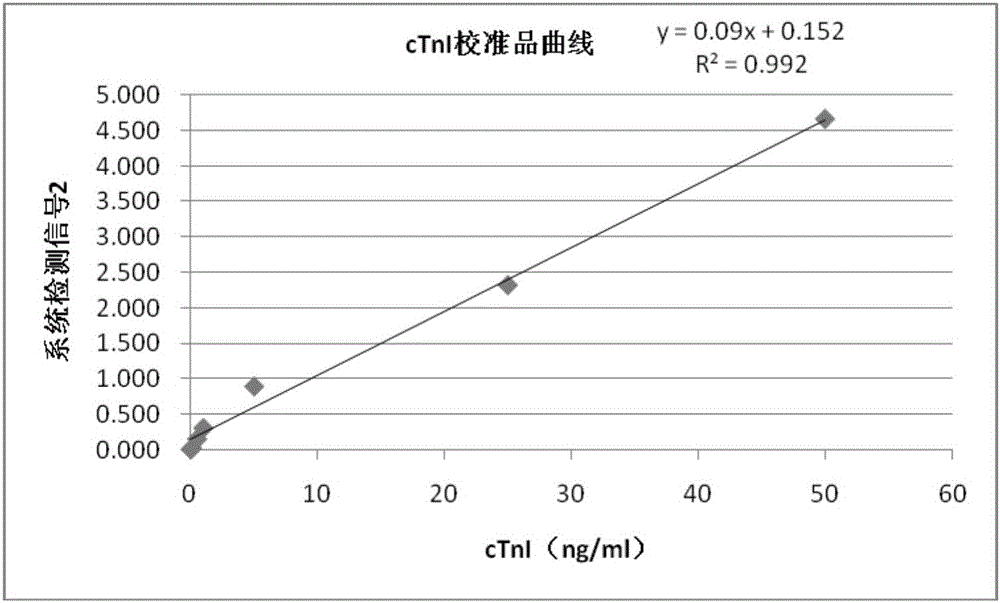
[0085] With the test method in embodiment 2, test the mixed standard substance of Myo, cTnI and CKMB respectively (the concentration of Myo is 100 and 400ng / ml, the concentration of cTnI is 1 and 25ng / ml, the concentration of CKMB is 10 and 50ng / ml) , and each standard product was repeatedly detected ten times, and the specific detection values are shown in Table 2 below.
[0086] Table 2 precision test result table
[0087]
[0088] As can be seen from the data in Table 2, using the triple detection test strip of the present invention, the precision of Myo, cTnI, and CKMB is all less than 10%, which fully meets the requirement that the precision of POCT products is less than 15%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com