Preparation method of chitosan quaternary ammonium salt
A technology of chitosan quaternary ammonium salt and chitosan, which is applied in the field of polymer modification, can solve the problems of poor water solubility and low reactivity, achieve good selectivity and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
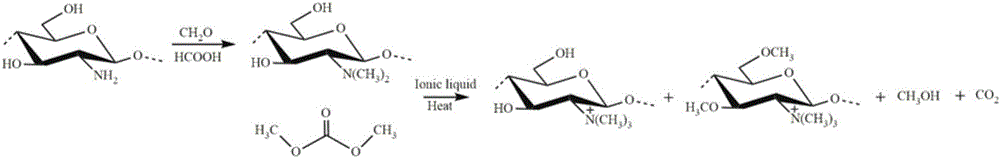
[0030] 5 grams of chitosan, 20 grams of formaldehyde, 20 grams of formic acid and 90 grams of distilled water were added into a three-necked flask, the magnetic stirring speed was 500 r / min, and the mixture was condensed and refluxed at 70°C for 120 hours to obtain a light yellow solution. The resulting light yellow solution was distilled under reduced pressure at 0.05MPa and 65°C for 25min, adjusted to pH=13 with sodium hydroxide solution, suction filtered at 0.05-0.1MPa, washed with absolute ethanol until neutral, and then washed with 1mol / L of hydrochloric acid to adjust the pH to 4, wash the obtained white precipitate and freeze-dry it at -30°C to obtain N, N-dimethyl chitosan;
[0031] Take 10 grams of (1-butyl-3-methylimidazole) chloride, add it into a 50 mL three-necked flask, turn on the magnetic stirrer and condensed water, and set the speed at 500 r / min. After the temperature reached 100°C, 0.5 g of N, N-dimethyl chitosan was added three times, and after stirring an...
Embodiment 2
[0034] N, the preparation method of N-dimethyl chitosan is with example 1;
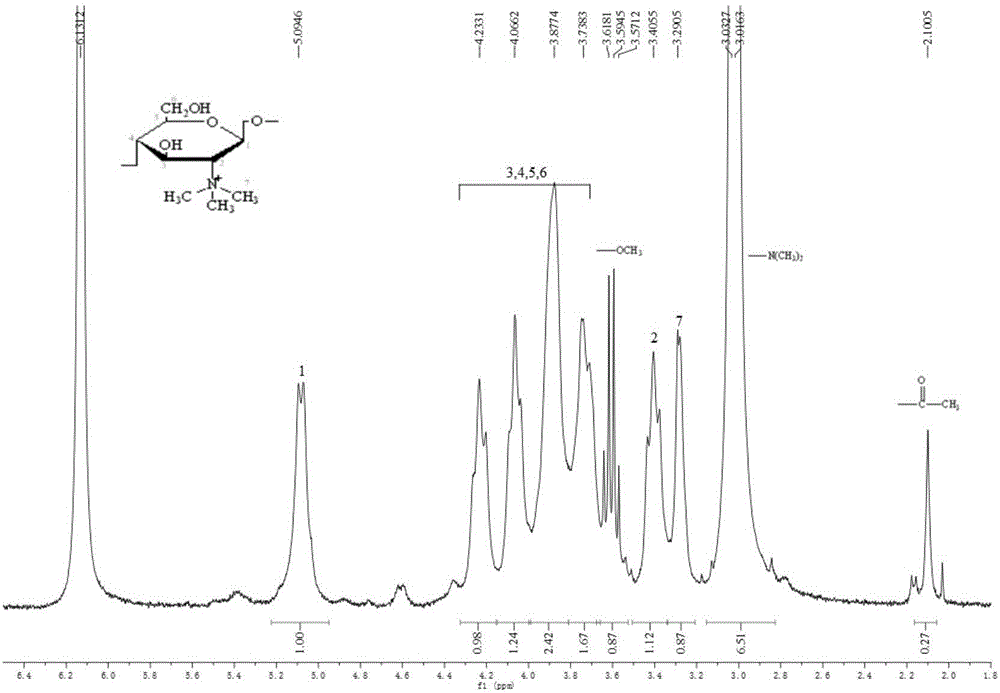
[0035] Take 5 grams of 1-ethyl-3-methylimidazole acetate, add it into a 50 mL three-necked flask, turn on the magnetic stirrer and condensed water, and set the speed at 600 r / min. After the temperature reached 90°C, 0.5 g of N, N-dimethyl chitosan was added three times, and after 2 hours of reaction, a uniform and viscous N, N-dimethyl chitosan mixture was obtained; Dimethyl carbonate (10 times excess) was added to the mixture, reacted at 130°C under reflux for 2.5 hours, the resulting solution was washed 5 times with absolute ethanol, and dried in an oven at 110°C, the obtained solid passed 1 H NMR and 13 C NMR analysis shows that the product is a chitosan quaternary ammonium salt with a quaternary ammonium degree of 8.78%.
Embodiment 3
[0037]N, the preparation method of N-dimethyl chitosan is with example 1;
[0038] Take 15 grams of 1-allyl-3-methylimidazole chloride and add it into a 50 mL three-necked flask, turn on the magnetic stirrer and condensed water, and set the speed at 400 r / min. After the temperature reached 110°C, 0.5 g of N, N-dimethyl chitosan was added three times, and after 2 hours of reaction, a uniform and viscous N, N-dimethyl chitosan mixture was obtained; 2.5 g Dimethyl carbonate (5 times excess) was added to the mixture, reacted under reflux at 170°C for 3 hours, the resulting solution was washed 6 times with absolute ethanol, and dried in an oven at 120°C, the obtained solid was N, N , N-trimethyl chitosan.
[0039] pass 1 H NMR and 13 C NMR analysis shows that the quaternary ammonium degree of the product chitosan quaternary ammonium salt is 12.39%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com