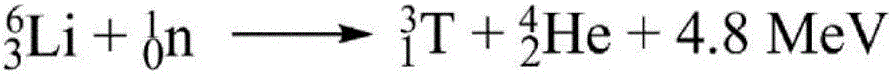Adsorbent for lithium isotope separation and preparation method and application thereof
A lithium isotope and adsorbent technology, applied in the field of adsorbents for lithium isotope separation, can solve the problems of complex process, large organic solvent, difficult stripping, etc., and achieve the effects of environmental friendliness and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
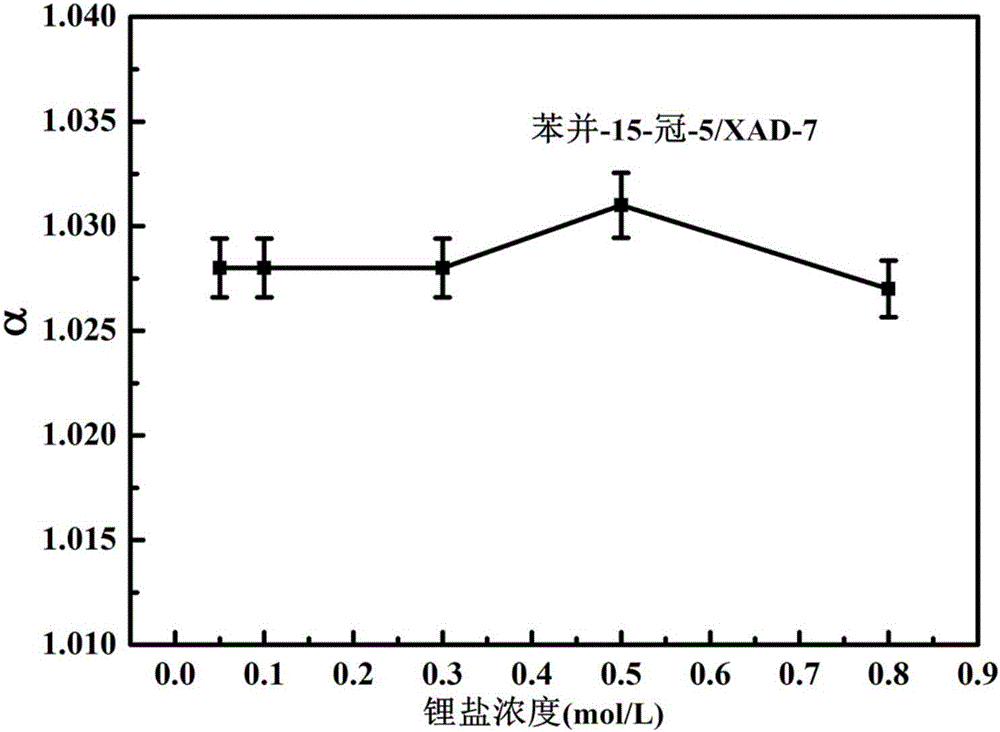
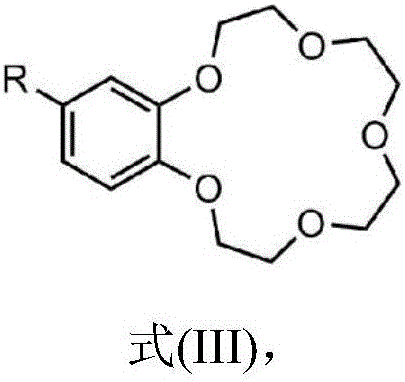
Image
Examples
Embodiment 1
[0029] (1) Pretreatment: wash the commercially available product XAD-7 with absolute ethanol for 3-5 times, and place it in a vacuum drying oven to dry overnight;
[0030] (2) Activation: Add 1.4g of XAD-7 into chloroform, sonicate at room temperature for 10 minutes, and then vibrate in a shaker for 10 minutes to obtain activated XAD-7;
[0031] (3) Dissolving and mixing: Weigh 0.6g of benzo-15-crown-5 into a 100mL round bottom flask, add 30mL of chloroform, ultrasonically dissolve benzo-15-crown-5 completely, and then mix with 1.4g of activated Mix and seal the porous polymer resin; then use a shaker to vibrate at 25°C for 24 hours; after the oscillation is over, move the system into a water bath and evaporate on a rotary evaporator at 25°C for 2 hours, then raise the temperature to 40°C, and continue Rotary evaporation for 1 hour, until the mixture was a loose powder, during this process, benzo-15-crown-5 entered the pores of the activated XAD-7 carrier through capillary act...
Embodiment 2
[0033] (1) Pretreatment: Wash commercially available XAD-7 3-5 times with absolute ethanol, and dry overnight in a vacuum oven;
[0034] (2) Activation: Add 1.2g of XAD-7 to nitrobenzene, sonicate for 10 minutes at room temperature, and shake in a shaker for 10 minutes to obtain activated XAD-7;
[0035] (3) Dissolving and mixing: Weigh 0.8g 4-nitrobenzo-15-crown-5 into a 100mL round bottom flask, add 30mL nitrobenzene, and ultrasonically make 4-nitrobenzo-15-crown-5 Completely dissolve, then mix with 1.2g activated porous polymer resin and seal well; then use a shaker to vibrate at 40°C for 30h; After steaming for 2 hours, raise the temperature to 80°C and continue rotary steaming for 1 hour until the mixture is a loose powder. During this process, 4-nitrobenzo-15-crown-5 enters into the activated XAD-7 carrier through capillary action The pores were finally dried at 50°C under vacuum for 24 hours to obtain a yellow and loose adsorbent.
Embodiment 3
[0037] (1) Pretreatment: commercially available Amberchrom TM CG-71 was washed 3-5 times and dried overnight in a vacuum oven;
[0038] (2) Activation: 1.0g Amberchrom TM CG-71 was added to 4-methyl-2-pentanone, sonicated at room temperature for 15 minutes, and then shaken in a shaker for 15 minutes to obtain the activated Amberchrom TM CG-71;
[0039] (3) Dissolving and mixing: Weigh 1.0g of 4-aminobenzo-15-crown-5 into a 100mL round bottom flask, add 30mL of 4-methyl-2-pentanone, and ultrasonically make 4-aminobenzo-15- Crown-5 was completely dissolved, then mixed with 1.0g of activated porous polymer resin and sealed; then shaken with a shaker at 30°C for 24h; After 2 hours of rotary evaporation under the same conditions, the temperature was raised to 50°C, and the rotary evaporation was continued for 1 hour until the mixture was a loose powder. During this process, 4-aminobenzo-15-crown-5 entered the activated Amberchrome through capillary action. TM In the pores of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com