Luminescent material and preparation method thereof, and organic light emitting diode using same
A technology of light-emitting diodes and light-emitting materials, which is applied in the direction of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve problems such as device efficiency limitations, and achieve high decomposition temperature, high yield, good solubility and film-forming properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
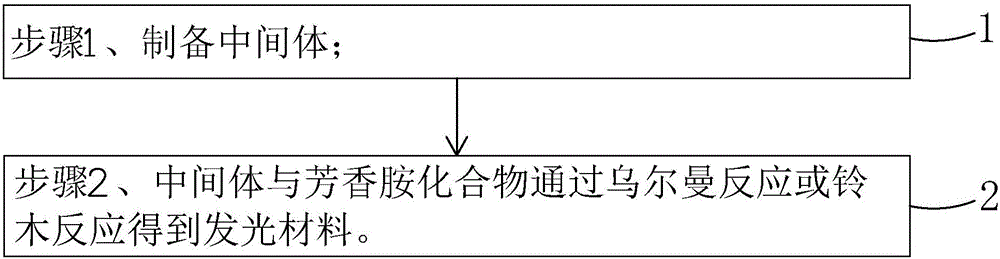
[0057] see figure 1 , the present invention also provides a method for preparing the above-mentioned luminescent material, comprising the following steps:
[0058] Step 1, prepare intermediate
[0059] The intermediate The synthetic route of is:
[0060]
[0061] Specifically, the step 1 includes:
[0062] Step 11, react m-bromothiophenol with 2-fluoro-4-bromobenzonitrile to obtain
[0063] The concrete implementation steps of described step 11 are:
[0064] In a 250ml three-necked flask, slowly add 0.73g (30mmol) NaH into 20ml of dry dimethylformamide (DMF) dissolved with 4.7g (25mmol) m-bromothiophenol, and then dropwise add 5g (25mmol) of NaH dissolved therein ) 2-fluoro-4-bromobenzonitrile in 20 ml of dry dimethylformamide. Under the protection of nitrogen, heat and reflux the reaction for 20h. After the reaction is completed, it is cooled to room temperature. The reaction solution is poured into 50ml of 1M NaOH solution, extracted with dichloromethane (DCM),...
Embodiment 1
[0090] Embodiment 1: intermediate Compound P6 was obtained by Ullmann reaction with carbazole.
[0091] The synthetic route of described compound P6 is as follows:
[0092]
[0093] The concrete implementation steps of described embodiment 1 are:
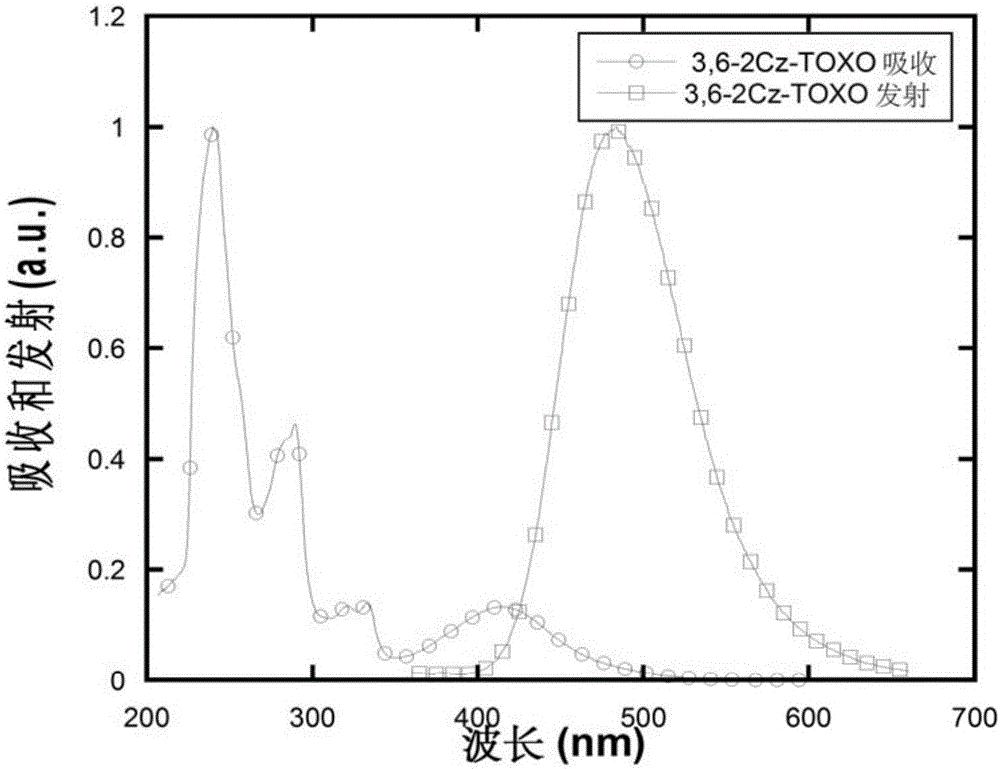
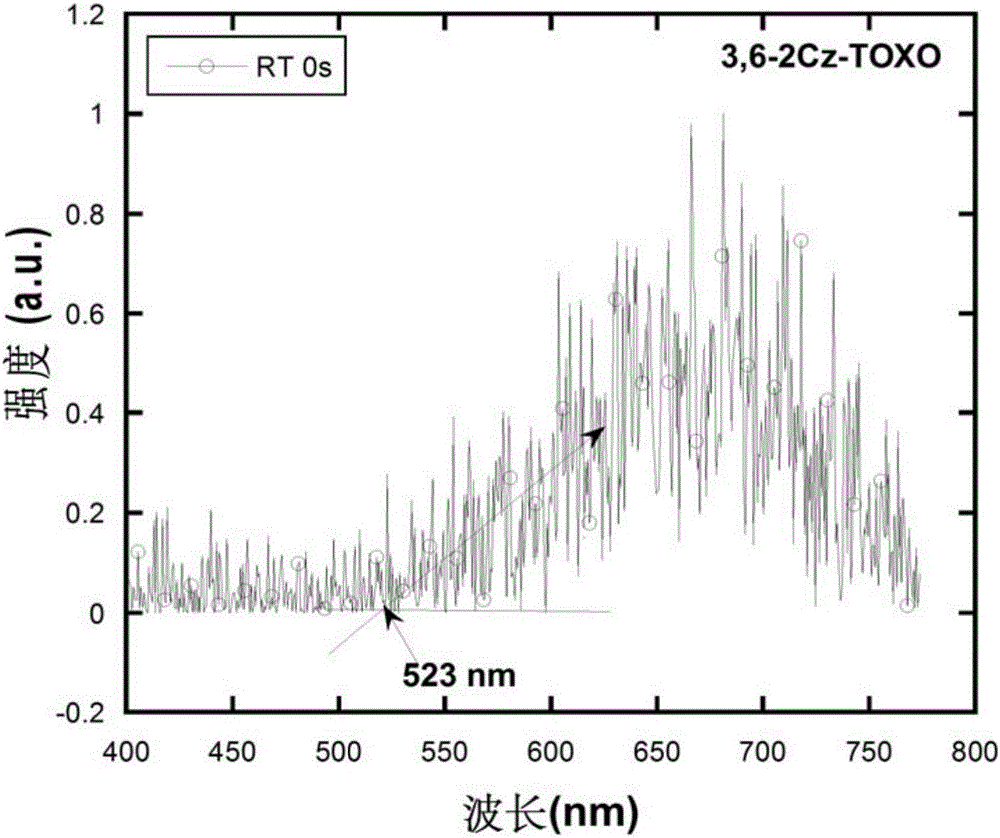
[0094] Under nitrogen protection, add 100ml toluene, 0.72g (2mmol) intermediate to the three-necked flask 0.67g (4mmol) carbazole, add 0.3g sodium tert-butyl alkoxide under stirring, then add 20mg tris(dibenzylideneacetone) dipalladium (Pd2(dba)3), then add 0.3ml 10% tri-tert-butyl Phosphine-n-hexane solution, heated to reflux, and reacted overnight. Cool down, extract the organic phase with dichloromethane, spin dry, and pass through the column. 0.77 g of white solid product was obtained, the yield was 67%. Molecular formula: C 37 h 22 N 2 o 3 S; M / Z=574.14; Theoretical values: 574.14 (100.0%), 575.14 (40.4%), 576.14 (9.2%), 576.13 (4.5%), 577.13 (1.8%), 575.13 (1.5%), 577.15 (1.0 %); elemental analysis: C, 77.33; H,...
Embodiment 2
[0096] Embodiment 2: intermediate Compound P10 was obtained by Ullmann reaction with 9,9-dimethylacridine.
[0097] The synthetic route of the compound P10 is as follows:
[0098]
[0099] The concrete implementation steps of described embodiment 2 are:
[0100] Under nitrogen protection, add 100ml toluene, 0.72g (2mmol) intermediate to the three-necked flask 0.84g (4mmol) 9,9-dimethylacridine, add 0.3g sodium tert-butyl alkoxide under stirring, then add 20mg tris(dibenzylideneacetone) dipalladium (Pd2(dba)3), then add 0.3ml of 10% tri-tert-butylphosphine-n-hexane solution was heated to reflux and reacted overnight. Cool down, extract the organic phase with dichloromethane, spin dry, and pass through the column. 0.78 g of white solid was obtained, yield 62%. Molecular formula: C 43 h 34 N 2 o 3S; M / Z=658.23; Theoretical values: 658.23 (100.0%), 659.23 (48.2%), 660.24 (10.8%), 660.22 (4.5%), 661.23 (2.2%), 661.24 (2.0%), 660.23 (1.3 %); elemental analysis: C, 78....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com