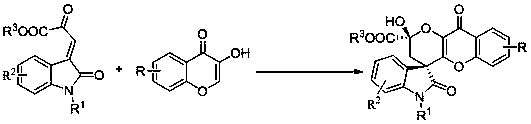
A kind of synthetic method of chiral spiro-oxindole-benzopyran-keto-3,4-dihydro-pyran compound
A technology for spirocyclic oxindole and pyran compounds, which is applied in the field of synthesis of chiral spirocyclic compounds, can solve the problems of limited application value, low diastereoselectivity, large amount of catalyst, etc., and achieves corresponding selectivity. Excellent, easy-to-use, easy-to-handle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
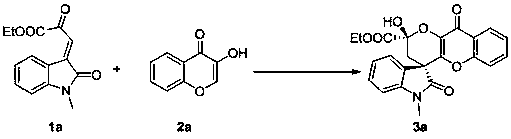
Embodiment 1
[0026]
[0027] Add quininethiourea (5.9 mg, 0.01 mmol), and 1a (25.9 mg, 0.1 mmol), 2a (16.2 mg, 0.1 mmol) sequentially into the reaction flask, add 1 mL of dichloromethane, and react at room temperature for 12 hours , the reaction system was subjected to simple column chromatography (eluent: petroleum ether: ethyl acetate = 1.8:1) to obtain the target product 3a (36.6 mg), a yellow solid, mp: 198-201 ° C, the yield was 87 %, >20 / 1 dr, 87% ee.
[0028] Add quinine thiourea (5.9 mg, 0.01 mmol) and 1a (25.9 mg, 0.1 mmol) and 2a (16.2 mg, 0.1 mmol) sequentially into the reaction flask, add 1 mL of dichloromethane, and react at zero degrees Celsius for 12 hours, the reaction system was subjected to simple column chromatography (petroleum ether: ethyl acetate = 1.8:1 as the eluent) to obtain the target product 3a (33.3 mg), a yellow solid, mp: 198-201 ℃, and the yield was 79%, >20 / 1 dr, 96% ee.
[0029] Add quinine thiourea (3.0 mg, 0.005 mmol) and 1a (25.9 mg, 0.1 mmol) succ...
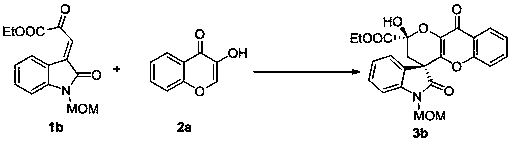
Embodiment 2
[0032]
[0033] Add quinine thiourea (3.0 mg, 0.005 mmol) and 1b (28.9 mg, 0.1 mmol) successively to the reaction flask, add 1 mL of dichloromethane, react at minus 20 degrees Celsius for 20 minutes, add 2a (24.3 mg, 0.15 mmol), continued to react for 40 hours, and the reaction system was subjected to simple column chromatography (eluent: petroleum ether: ethyl acetate = 1.6:1) to obtain the target product 3b (42.8 mg), yellow solid, mp: 141 -144 ℃, the yield is 95%, >20 / 1 dr, 98% ee.
[0034] Product 3b is analyzed, and the results are as follows: Measure [Daicel Chiralpak IA, hexane / i -PrOH (70:30), flow rate: 1.0 mL min -1 , λ = 254 nm, t(major) = 12.362, t(minor) = 17.090]; [α] D 25 = + 98.0 (c = 0.50, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ8.48 (s, 0.3H), 8.22 (d, J = 7.2 Hz, 0.4H), 8.18 (d, J = 7.6 Hz, 0.6H), 7.93(d, J = 7.6 Hz, 0.6H), 7.51 – 7.49 (m, 1H), 7.42 (t, J = 7.2 Hz, 0.3H), 7.36(t, J = 7.6 Hz, 0.7H), 7.33 – 7.28 (m, 1H), 7.23 (d, J = 2.9 H...
Embodiment 3
[0036]
[0037] Add quininethiourea (0.002 mmol) and 1c (33.5 mg, 0.1 mmol) to the reaction flask in turn, add 1 mL of dichloromethane, react at minus 20 degrees Celsius for 20 minutes, add 2a (24.3 mg, 0.15 mmol) , continued to react for 38 hours, and the reaction system was subjected to simple column chromatography (eluent: petroleum ether: ethyl acetate = 1.8:1) to obtain the target product 3c (48.3mg), a white solid, mp: 141-144 ° C, the yield is 91%, >20 / 1 dr, 96% ee.
[0038] Add quinine thiourea (3.0 mg, 0.005 mmol) and 1c (33.5 mg, 0.1 mmol) in turn to the reaction flask, add 1 mL of dichloromethane, react at minus 20 degrees Celsius for 20 minutes, add 2a (24.3 mg, 0.15 mmol), continued to react for 36 hours, and the reaction system was subjected to simple column chromatography (eluent: petroleum ether: ethyl acetate = 1.8:1) to obtain the target product 3c (48.7 mg), white solid, mp: 141 -144 ℃, 98% yield, >20 / 1 dr, 99% ee.
[0039] Product 3c is analyzed, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com