Method for preparing industrial safinamide mesylate
A technology of safinamide mesylate and propionamide, which is applied in the field of industrialized preparation of safinamide mesylate, can solve the problems of complicated operation, high cost, large environmental pollution and the like, and achieves simple post-processing and low cost , Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
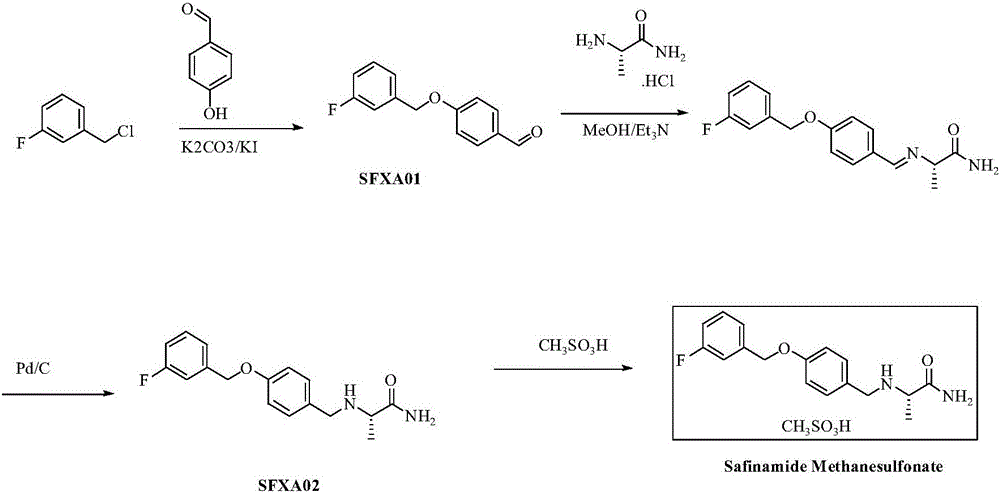
[0013] 1. Preparation of 4-(3-fluorobenzyloxy)benzaldehyde (SFXA01):
[0014] Add 15.2g (124.5mmol) of p-hydroxybenzaldehyde, 17.2g (124.5mmol) of potassium carbonate and 2.0g (12mmol) of potassium iodide to 170ml of absolute ethanol, stir for 15min, and add 17.3ml (141.7mmol) of 3-fluorochlorobenzyl dropwise. ), heated to reflux for 6h. Filter, wash the filter cake with absolute ethanol (15ml×3), combine the filtrate and lotion, concentrate under reduced pressure, add 60ml of toluene and 20ml of water to the remaining light yellow oil, stir for 30min, let stand to separate layers, and separate the toluene layer , concentrated under reduced pressure, and the residue was recrystallized from toluene-n-hexane (1:1) to obtain 26.1 g (91%) of 4-(3-fluorobenzyloxy)benzaldehyde as a white solid, mp 43.8-43.9°C.
[0015] 2. Preparation of (S)-2-[4-(3-fluorobenzyloxy)benzylamino]propionamide (SFXA02):
[0016] (S)-2-alanine methyl ester hydrochloride 15.4g (110mmol), 4-(3-fluorobenzy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com