A kind of preparation method of 1,5-cyclooctadiene iridium chloride dimer
A technology of cyclooctadiene iridium chloride and cyclooctadiene is applied in the field of preparation of 1,5-cyclooctadiene iridium chloride dimer, which can solve the problem of high purity and yield of dimer, and high reaction time. It can reduce the production cost, reduce the loss of waste liquid treatment, and reduce the amount of use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The invention provides a method for preparing 1,5-cyclooctadiene iridium chloride dimer. The method comprises: under anaerobic conditions, iridium-containing chlorine-containing compounds, aldehydes, 1,5-cyclooctadiene React with solvents.
[0019] According to the method of the present invention, the oxygen-free condition can be ensured by introducing an inert gas replacement into the reactor, and the number of replacements is not particularly limited, preferably 3-5 times, and the inert gas can be nitrogen, argon, At least one of helium and neon, preferably argon.
[0020] According to the present invention, preferably, the weight ratio of the iridium-containing chlorine-containing compound to the solvent is 1:5-20, and more preferably, the weight ratio of the iridium-containing chlorine-containing compound to the solvent is 1:5-10. In the preparation method of 1,5-cyclooctadiene iridium chloride dimer provided by the prior art, the amount of solvent used is large, a...
Embodiment approach
[0027] According to a preferred embodiment of the present invention, the method further includes: cooling, washing, filtering and drying in sequence after the contact reaction. The cooling, filtering, washing and drying can all be carried out according to conventional means in the art.
[0028] According to the present invention, preferably, the cooling is to naturally cool down to room temperature, and then transfer to an ice-water bath. Adopting this preferred embodiment is more conducive to the precipitation of products.
[0029] In the present invention, the filtration may be suction filtration.
[0030] In the present invention, the washing may be to sequentially use water and cold methanol to wash the filter cake obtained by suction filtration.
[0031] The present invention has no special limitation on the drying, and the drying can be carried out at room temperature under vacuum.
[0032] The present invention has no particular limitation on the embodiment of the con...
Embodiment 1
[0040] This example is used to illustrate the preparation method of the 1,5-cyclooctadiene iridium chloride dimer provided by the present invention.
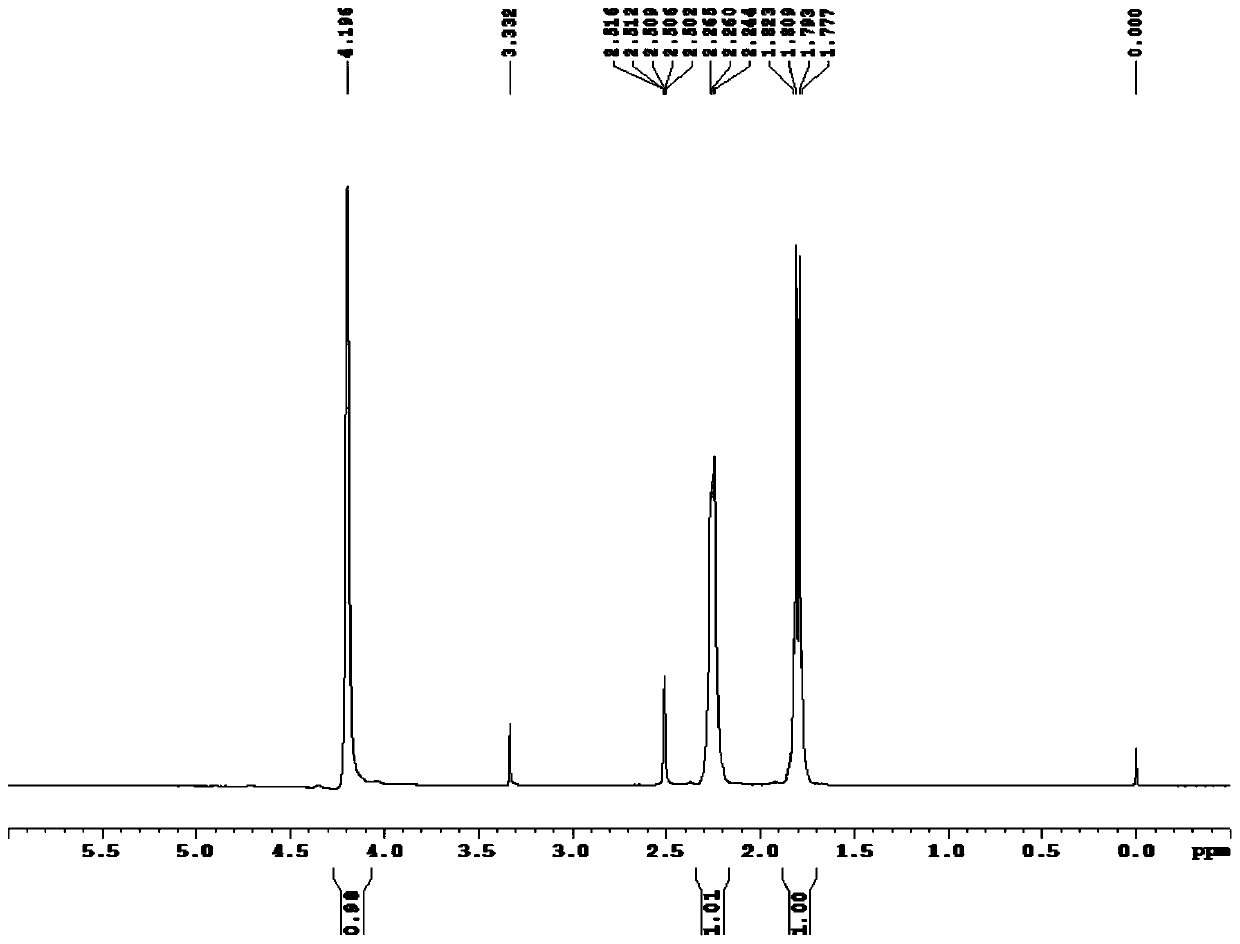
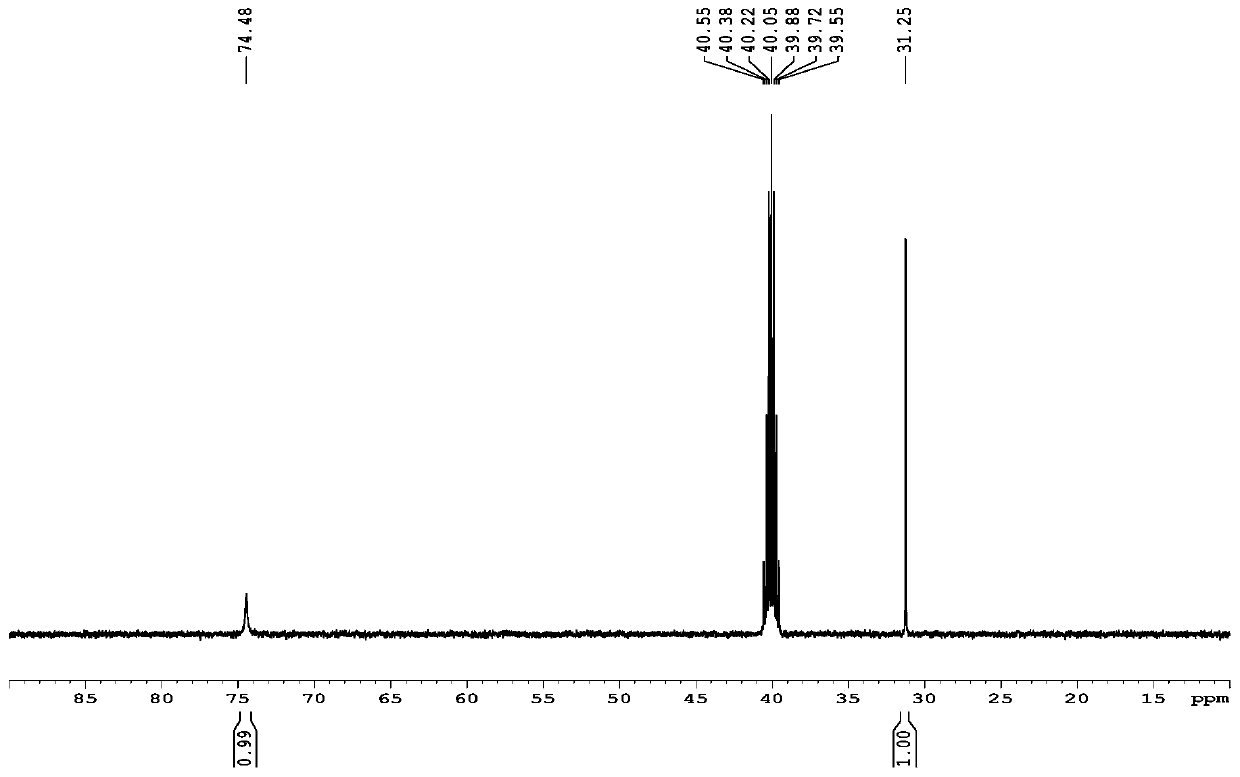
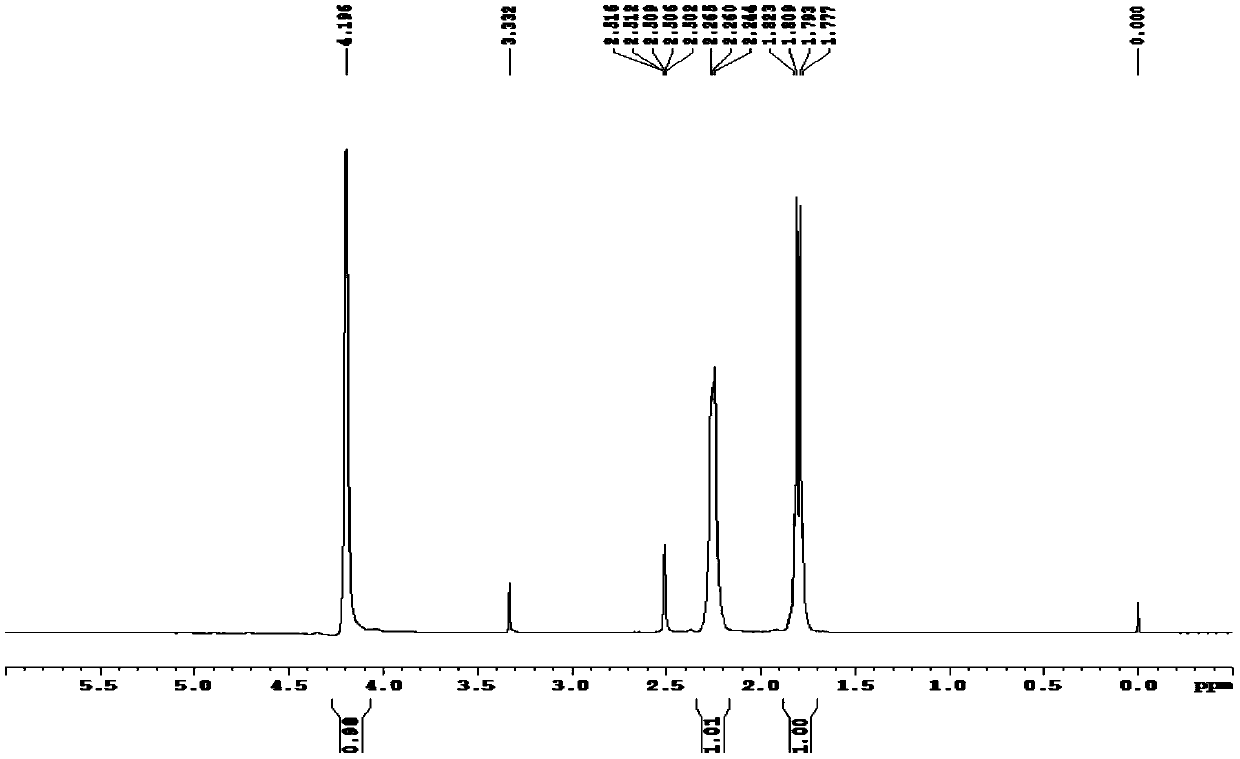
[0041] At room temperature (25°C), add 1 g of iridium trichloride monohydrate (3.1 mmol) with a mass content of 98% and 5 g of deoxygenated water (obtained by bubbling argon for 10 min) into a reaction flask filled with argon, and depressurize Replace argon 3 times, heat up to 80°C, add 1.38g of 40% acetaldehyde aqueous solution (acetaldehyde 12.5mmol), 1.4g of 98% 1,5-cyclooctadiene (12.7mmol) , reflux at 80°C for 6h, cool the reaction mixture to room temperature, transfer to an ice-water bath (0°C) for 1h, then perform suction filtration (2°C), wash with water (1g water, 1g cold methanol rinse) And drying (room temperature 25° C., 6 h), a red solid 0.77 g was obtained. The measured yield was 74%. The red solid is analyzed and determined by proton nuclear magnetic resonance spectroscopy, and the melting point of the red solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com