Boron-containing solid polymer electrolyte and preparation method and application thereof
A solid polymer, electrolyte technology, applied in the direction of hybrid capacitor electrolytes, circuits, fuel cells, etc., can solve the problems of explosive, poor transmission performance, unsatisfactory processing performance and other problems, achieve high degree of dissociation, excellent cycle performance, The effect of a wide electrochemical stability window
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 21.75 mmol of bis(trifluoromethyl)phenylboronic acid (BPBA), 21.75 mmol of 3-allyloxy-1,2-propanediol (GAE), 20 g of 4Å molecular sieves, and 200 ml of anhydrous dichloromethane into a 500 ml three-port In the beaker, pass inert gas for protection, and keep stirring the solution for more than 24h. The mixture was suction-filtered more than 3 times, and then washed with a neutral alumina column to obtain a dichloromethane solution of pure alkene boron-containing heterocyclic monomer (GAE-BPBA). After rotary evaporation, the pure light yellow monomer (GAE-BPBA) was obtained, and its yield was approximately equal to 1.
[0034] Take 2ml of the above-mentioned monomer GAE-BPBA, the content of the initiator (BPO) is 0.5%-1%, and 20ml of solvent N,N-dimethylformamide (DMF) into a 50ml three-necked flask, under the protection of inert gas, After free radical reaction at 85°C for 6-8h, the resulting mixture was precipitated with anhydrous petroleum ether to obtain a white ...
Embodiment 2
[0036]Add 21.75 mmol of bis(trifluoromethyl)phenylboronic acid (BPBA), 21.75 mmol of 3-allyloxy-1,2-propanediol (GAE) and 100 ml of anhydrous toluene into the In a 200ml three-necked beaker, remove water azeotropically for 6-8 hours at a temperature of 130-145°C. The mixture was suction-filtered more than 3 times, and then washed with a neutral alumina column to obtain a pure alkene boron-containing heterocyclic monomer (GAE-BPBA) in toluene. After rotary evaporation, the pure light yellow monomer (GAE-BPBA) was obtained, and its yield was approximately equal to 1.
[0037] Take 2ml of the above-mentioned monomer GAE-BPBA, the content of the initiator (BPO) is 0.5%-1%, and 20ml of solvent N,N-dimethylformamide (DMF) into a 50ml three-necked flask, under the protection of inert gas, After free radical reaction at 85°C for 6-8h, the resulting mixture was precipitated with anhydrous petroleum ether to obtain a white powder, which was dried in vacuum at 40°C for 24h to obtain dry...
Embodiment 3
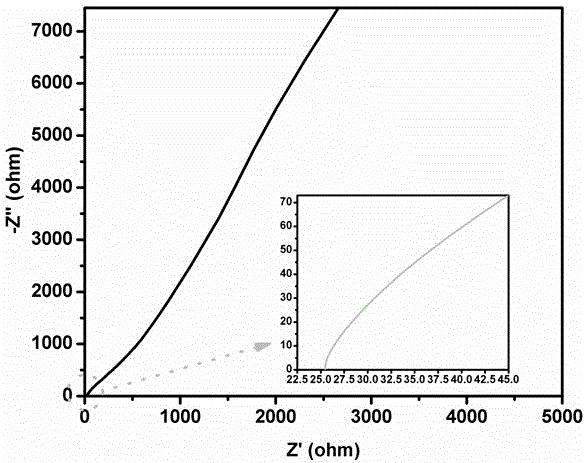
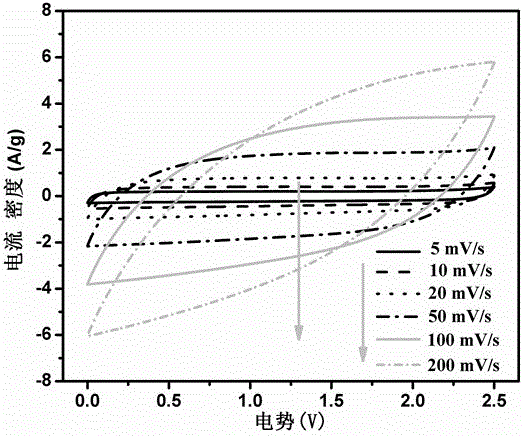
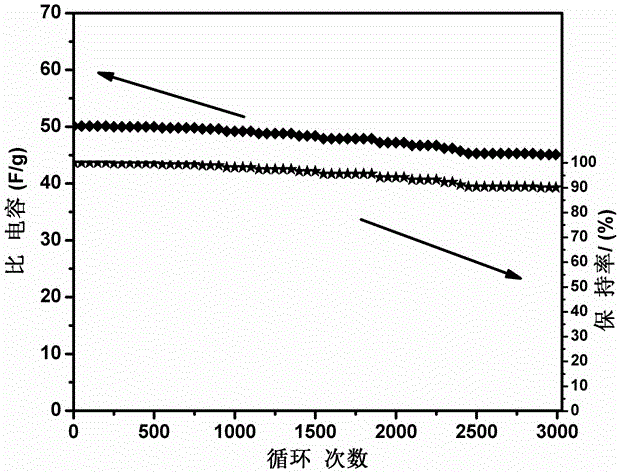
[0039] Accurately weigh 2ml of GAE-BPBA containing boron heterocycloalkene monomer, and the electrolyte solution is 2M LiClO 4 DMF solution, the amount of lithium perchlorate added is 10% of the monomer mass fraction, and the content of initiator (BPO) is 0.5%-1%. After stirring evenly at room temperature (2-3h), cast the mixture into a 0.5mm thick PTFE mold, then place it in a high-purity nitrogen-protected drying oven, and react at 85°C for 6-8h. After cooling to room temperature, the obtained polymer film was placed in a freeze dryer, and traces of organic solvents were removed at -80°C for more than a week. Finally, a boron-containing solid polymer electrolyte is obtained, and the thickness of the film is 150-200 μm. The conductivity of the polymer electrolyte was measured using a sandwich structure that was assembled in a 2016 button cell in the order of stainless steel / polymer electrolyte / stainless steel. The AC impedance spectrum of the polymer electrolyte was tested,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com