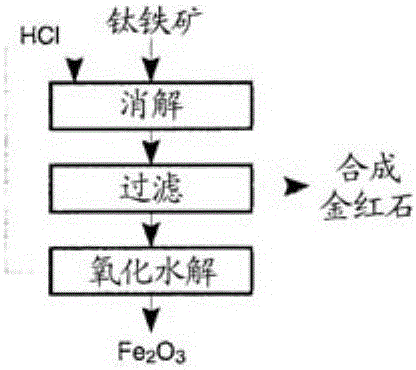
The production of high-grade synthetic rutile from low-grade titanium-bearing ores
An ore, low-level technology, used in the field of two-stage leaching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
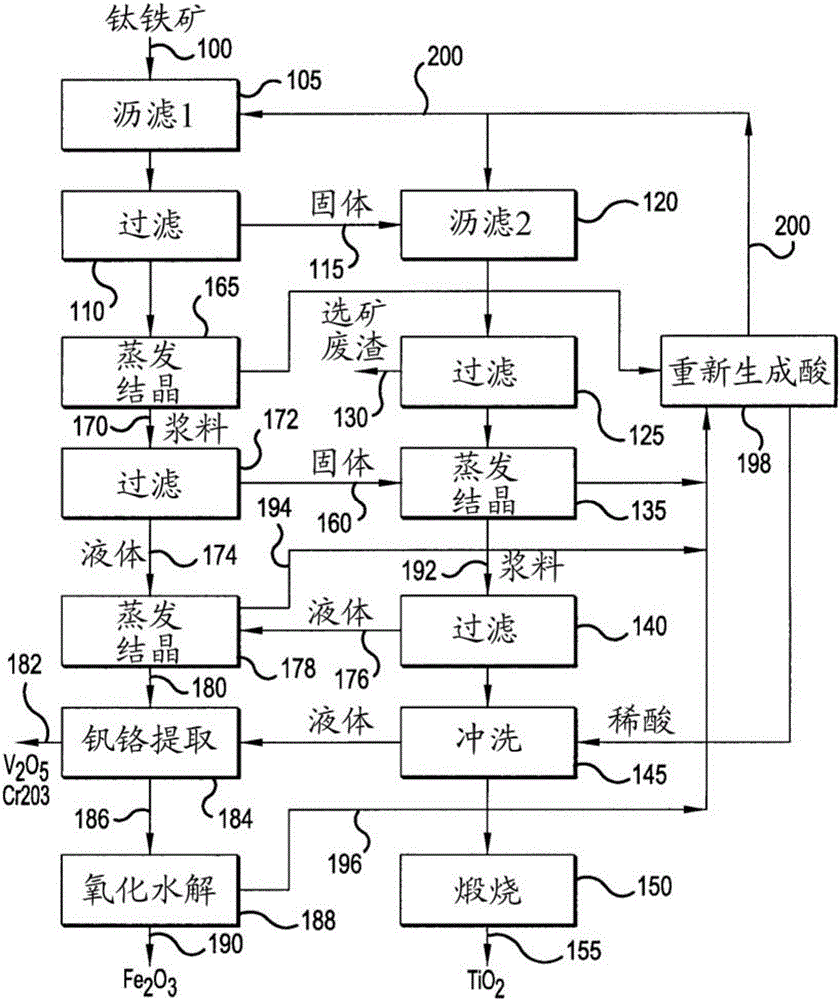
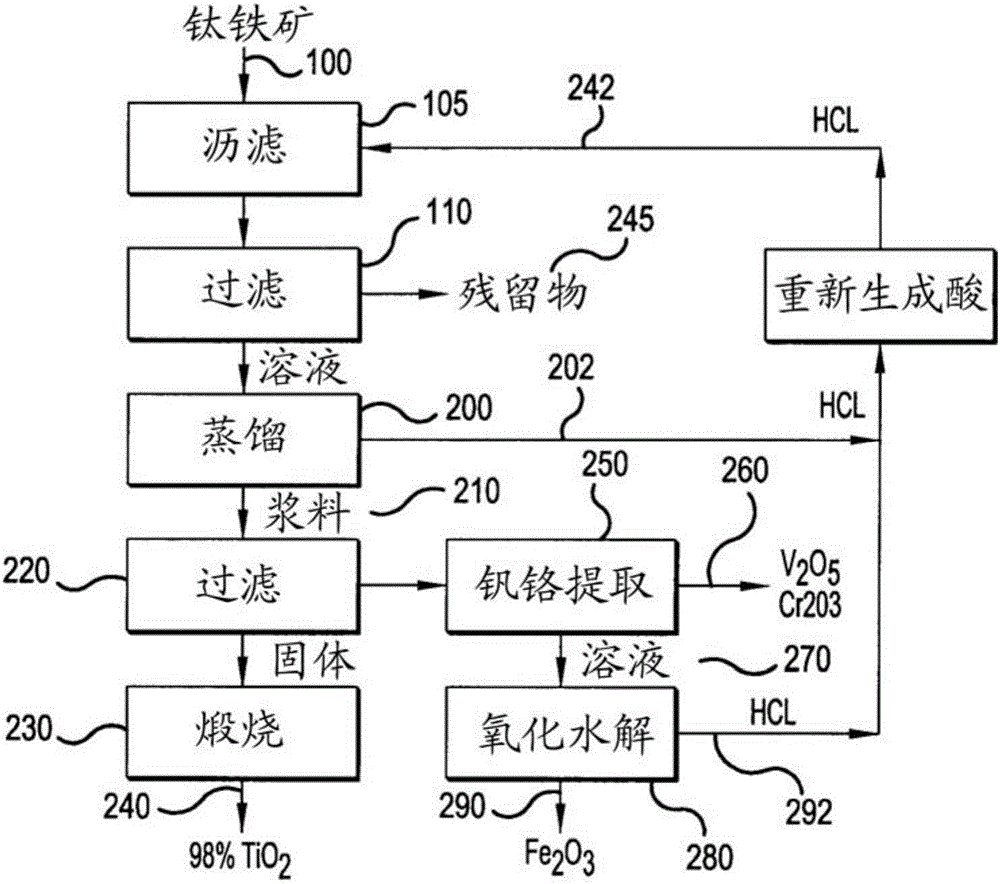
[0046] [Example 1] Low-grade ore (containing 11% TiO2) from Magpie deposits in Quebec, Canada 2 ) Dissolution and removal of insoluble substances.
[0047] The first stage:
[0048] a. The low-grade ore is finely ground to 200 mesh, wherein preferably 50% of the range and more preferably 80% of the low-grade ore pass through -200 mesh respectively.
[0049] b. The first leaching reaction is carried out by contacting the low grade ore with hydrochloric acid at a concentration in the range of 35%-40%, using an ore to acid ratio between 2 and 2.5. Due to the pulp concentration and fine particle size determination, only slight agitation is required to prevent settling. This first leaching reaction dissolves the magnetite in about an hour. The temperature is kept at 60°C-70°C.
[0050] c. The mother liquor currently containing only 2%-4% HCl is preferably replaced by concentrated acid on hand to dissolve ilmenite and titanium present in the ore to obtain a slurry. The slurry i...
Embodiment 2
[0056] [Example 2] Precipitation of TiO(OH) by distillation of unreacted acid 2 .
[0057] second stage:
[0058] The two leaching reactions discussed in Example 1 consumed more than half of the available acid. Recovery of free unreacted acid was performed by mixing the two filtered solutions from The first leaching reaction and the second leaching reaction are discussed in Example 1. About 90% of the titanium chloride precipitates as hydrate. Another filtration step removes residual saturated liquid.
[0059] Chloride crystals are dissolved using a minimal amount of dilute acid, leaving insoluble TiO(OH) in the form of a fine granular solid 2 , which is easy to filter. After the calcination process, the advanced synthetic rutile contains TiO 2 The amount is 95%-98% TiO 2 range, which meets the requirements for synthetic rutile concentrates.
Embodiment 3
[0060] [Example 3] Recovery of combined hydrochloric acid and production of iron or iron oxide.
[0061] The third stage:
[0062] There are several possible ways to recover the iron and bound hydrochloric acid including:
[0063] 1. Reduction of ferric chloride with iron and partial evaporation of the solution to crystallize hydrated ferrous chloride, which can then be reduced to metal by hydrogen to produce iron powder.
[0064] 2. Send the chloride solution into the spray reactor under high temperature and hydrogen atmosphere. Production of iron powder and simultaneous regeneration of hydrochloric acid and evaporated water. The iron produced contains 0.4% TiO 2 and 1%-3.5% Cr 2 o 3 .
[0065] 3. Feed the chloride solution into a spray reactor where high temperature hydrolysis in a slightly oxidizing atmosphere produces iron oxides and hydrochloric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com