Nano-scale solid-state lipid drug delivery system and preparation method thereof
A drug-carrying system and solid lipid technology, applied in the field of chemical industry, can solve the problem that there is no report on the drug-carrying system of 4-n-n-butylresorcinol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1) According to the preparation method involved in the summary of the invention, the following method
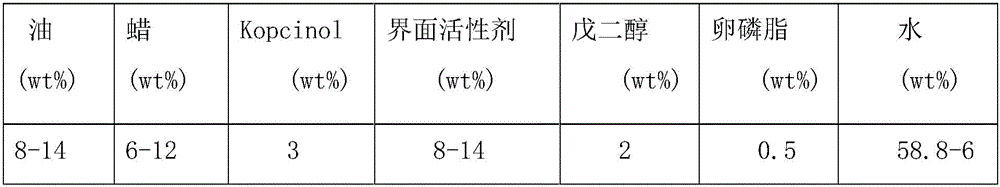
[0075] 1) Turn on the cooling water of the high-pressure homogenizer, turn on the heater of the high-pressure homogenizer to heat to 85°C; start the high-pressure homogenizer, and keep the circulation to keep the temperature of the pipeline in the machine at 85°C; weigh all the medicines separately, and put Liquid lipids, solid lipids and active drugs are mixed and heated to 85°C to obtain an oil phase;
[0076] 2) Mixing water, surfactant, polyol and phospholipid and heating at constant temperature to 85°C to obtain the water phase;
[0077] 3) Add the oil phase in step 1) to the water phase in step 2), and keep the temperature at 85°C to obtain a mixed phase;
[0078] 4) Put the mixed phase in step 3) into a high-pressure homogenizer for pre-emulsification at a speed of 8000 rpm for 180 seconds to form a mixed phase emulsion and obtain a sample;
[0079] 5) Feed t...
Embodiment 2
[0084] Preparation method is identical with embodiment 1,
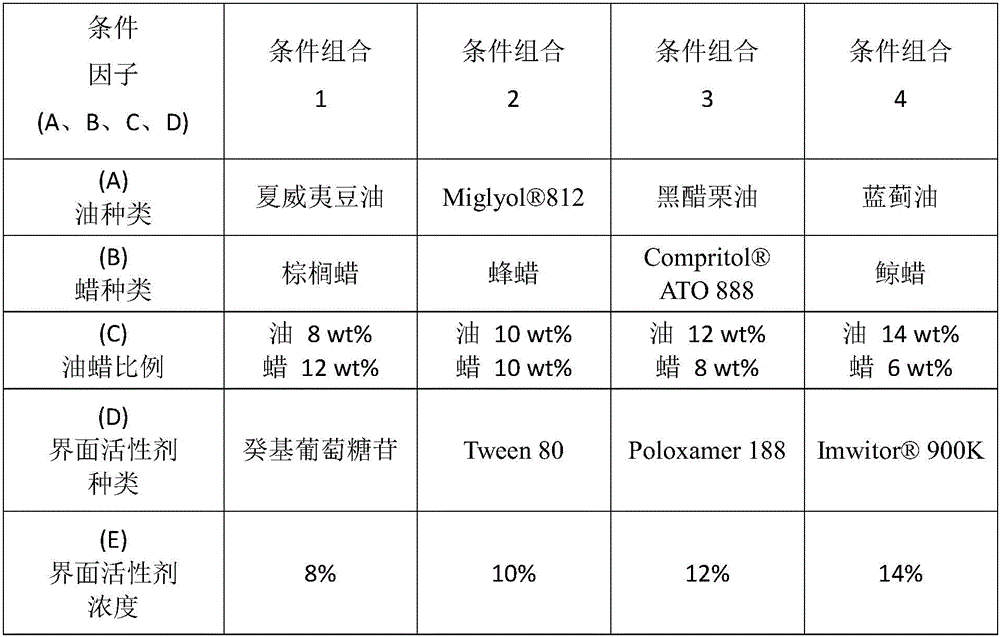
[0085] The liquid lipid is 10% macadamia oil, the solid lipid is 10% beeswax, the active drug is 4-n-butylresorcinol 3%, the surfactant is Tween 80, 2% The polyhydric alcohol is pentylene glycol, the 0.5% phospholipid is lecithin, and the rest is water, and the above fractions all refer to mass fractions. Get Q2.
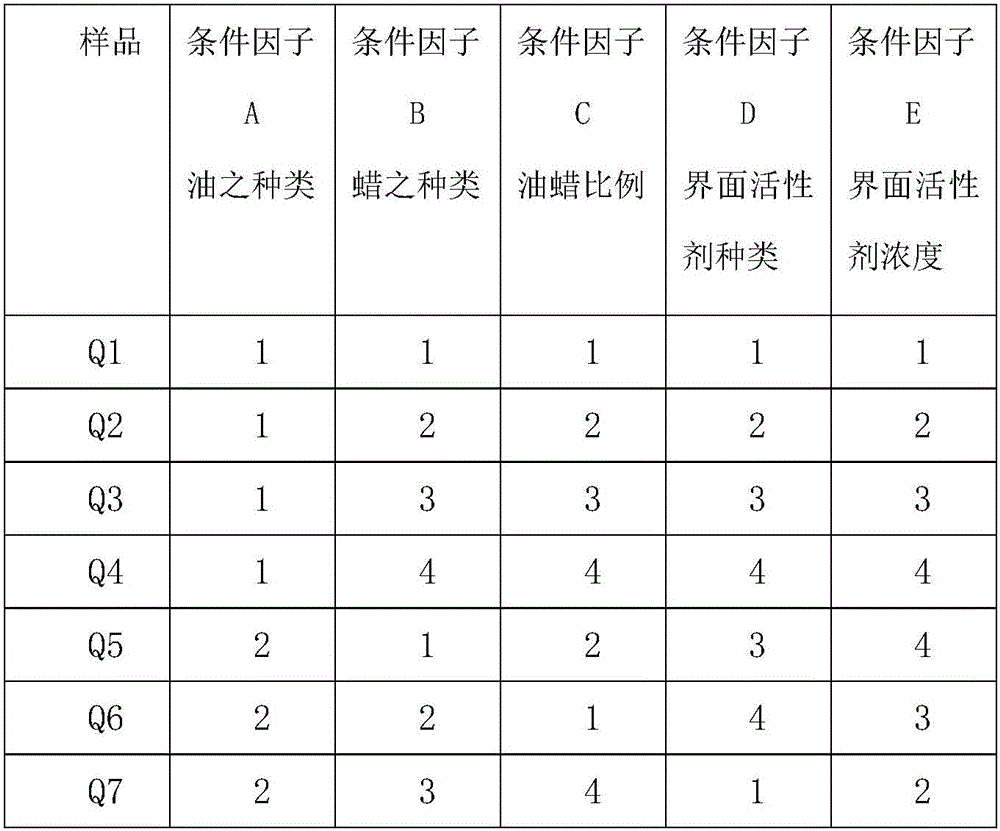
[0086] For particle size measurement, see row Q2 in Table 4, see Table 5Q2 for interface potential, see 0.7% of Q2 in Table 6 for crystallinity, and 91.94% of Q2 in Table 7 for embedding degree.
Embodiment 3
[0088] Preparation method is identical with embodiment 1,
[0089] Among them, the liquid lipid is 12% Hawaiian soybean oil, and the solid lipid is 8% ATO 888, 3% active drug is 4-n-butylresorcinol, 12% surfactant is Poloxamer 188, 2% polyol is pentylene glycol, 0.5% phospholipid is lecithin, the rest is water, and the above fractions all refer to mass fractions. Get Q3.
[0090] For particle size measurement, see row Q3 in Table 4, see Table 5Q3 for interface potential, see 14.4% of Q3 in Table 6 for crystallinity, and 91.24% of Q3 in Table 7 for embedding degree.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| embedding rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com