Composite catalyst for catalyzing formaldehyde decomposition and preparation method thereof
A composite catalyst and a formaldehyde decomposition technology are applied to the composite catalyst for catalyzing formaldehyde decomposition and the field of preparation thereof, and can solve the problems of difficult catalytic decomposition efficiency, low catalytic efficiency, few active sites and the like, and achieve low cost, high catalytic activity, Simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
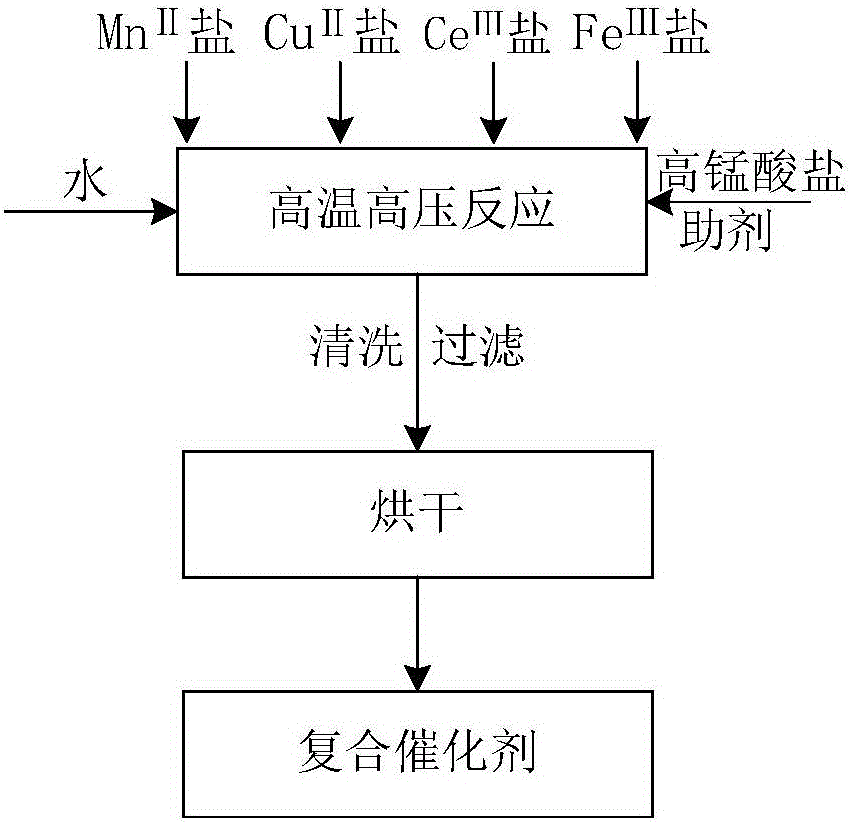
[0032] Add 113.31g of potassium permanganate, 56.37g of manganese sulfate monohydrate, 2.0g of copper nitrate trihydrate, 13.51g of ferric chloride hexahydrate and 2.1g of cerium sulfate tetrahydrate into 200mL of pure water, and stir until fully dispersed and dissolved , Add 12.44g potassium chloride until fully dissolved. The above mixed solution was transferred to a high-pressure reactor, set to react at 150°C for 10 hours, then taken out, washed and filtered, and then dried at 120°C for 25 hours to obtain the product.
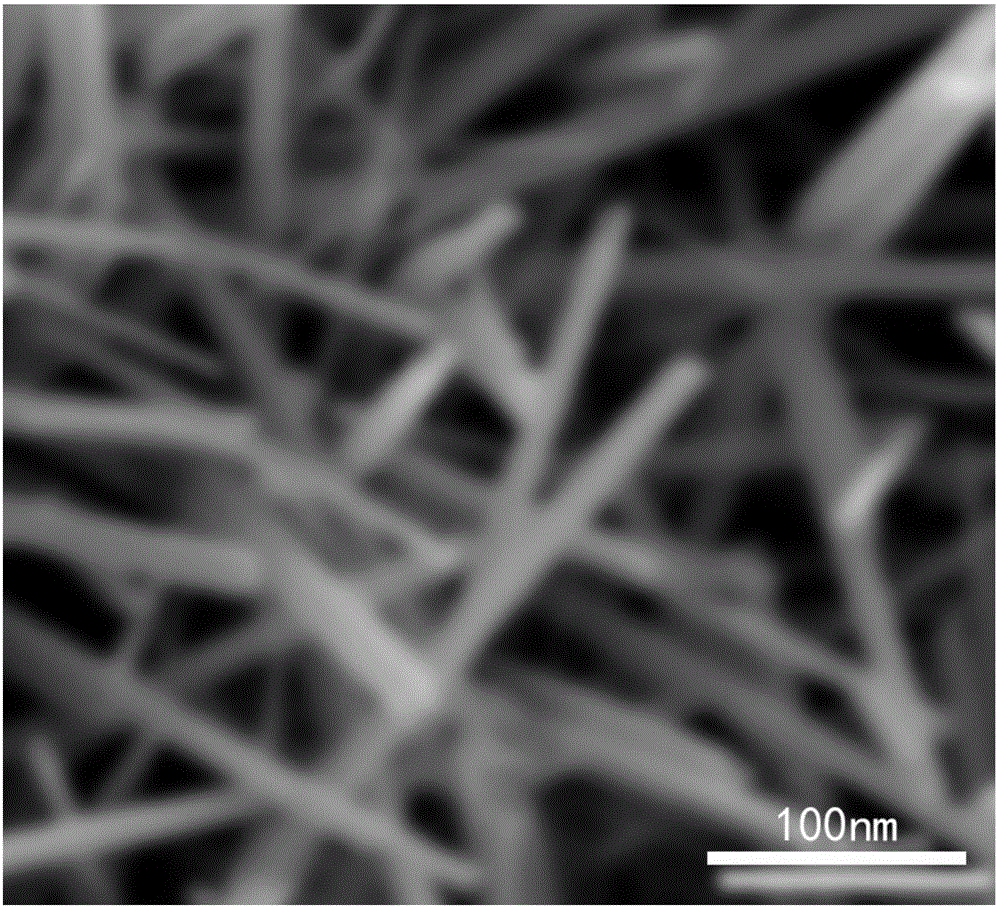
[0033] SEM tested (see figure 2 ), indicating that the synthesized ultra-dispersed catalytic formaldehyde decomposition composite catalyst material is a nano-rod material, and the diameter of the nano-rod is about 10nm.
[0034]Take 1.00 g of the above-prepared dispersed catalytic formaldehyde decomposition composite catalyst material and place it on a sand core in a glass tube with a diameter of 2 mm to test its catalytic performance. The air pump is re...
Embodiment 2
[0036] Add 126.48g of potassium permanganate, 95.73g of manganese nitrate hexahydrate, 8.34g of copper sulfate pentahydrate, 6.74g of ferric nitrate nonahydrate and 23.6g of cerium chloride hexahydrate in 200mL of pure water, and stir until fully dispersed and dissolved , Add 13.49g potassium nitrate until fully dissolved. Transfer the above mixed solution to a high-pressure reactor, set it to react at 120°C for 30 hours, take it out, wash and filter it, and then dry it at 180°C for 15 hours to obtain the product.
[0037] The catalytic performance test is the same as in Example 1, and the test results show that the composite catalyst material prepared in this example has an efficiency of 98% at room temperature for catalytically decomposing 1000 ppm of formaldehyde once.
Embodiment 3
[0039] Add 118.26g of potassium permanganate, 56.37g of manganese sulfate monohydrate, 28.43g of copper chloride dihydrate, 0.88g of ferric sulfate heptahydrate and 18.1g of cerium nitrate hexahydrate in 200mL of pure water, and stir until fully dispersed and dissolved , Add 11.62g of potassium sulfate until fully dissolved. The above mixed solution was transferred to a high-pressure reactor, set to react at 200°C for 1 hour, then taken out, washed and filtered, and then dried at 110°C for 30 hours to obtain the product.
[0040] The catalytic performance test is the same as in Example 1. The test results show that the efficiency of the composite catalyst material prepared in this example to catalyze and decompose 1000 ppm of formaldehyde once at room temperature is 98.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com