Method for preparing (R)-phenylglycol (PED) from racemic styrene oxide (rac-SO) under catalytic actions of double enzymes
A technology of racemic styrene oxide and styrene glycol, which is applied in the field of biocatalysis, can solve the problems of low substrate solubility, catalytic substrate concentration, catalytic efficiency, low enantiomeric purity of product yield, and enzyme-to-substrate Specific effects and other issues, to achieve the effect of increasing concentration, large industrial application potential and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] See GenBank: KR013755.1 for the nucleic acid sequence of the gene encoding VrEH3. The construction steps of E.coli / VrEH3 engineering bacteria expressing VrEH3 are as follows: synthesize the target gene, use pET-28a as the expression vector, and use E.coli BL21(DE3) as the expression host to construct the recombinant strain E.coli / VrEH3. Pick a single colony of E.coli / VrEH3 and culture it in 2mL LB medium at 37°C for 12h, then transfer it to fresh 100mL LB medium with an inoculum size of 2% (v / v), and culture it at 37°C After 2h, add the inducer IPTG to a final concentration of 0.05mM, incubate at 35°C for 10h, induce the high expression of VrEH3, collect the bacteria by centrifugation at 8000rpm, add 100mM, pH 7.0 phosphate buffer according to the ratio of 1g wet bacteria to 10mL buffer The suspension was prepared into a bacterial suspension with a bacterial concentration of 100 mg / mL and stored for later use.
[0021] For the amino acid sequence of AuEH2 and the const...
Embodiment 2
[0023] Determination of the specific enzyme activity of VrEH3 whole cells: Add 800 μL of VrEH3 bacterial suspension and 150 μL of potassium phosphate buffer (100 mM, pH=7.0) to a 1.5 mL EP tube, preheat at 25°C for 5 minutes; add 50 μL of 200 mM rac-SO After reacting for 15 minutes, 100 μL was extracted in 1 mL ethyl acetate containing (1 mg / mL n-hexanol as internal standard), dried over anhydrous magnesium sulfate, and passed through a 0.22 μm organic membrane.
[0024] AuH2 A250I Determination method of specific enzyme activity: add 33μLAuEH2 to 1.5mL EP tube A250I Bacteria suspension and 917 μL potassium phosphate buffer solution (100 mM, pH=7.0), preheated at 35°C for 2 min; add 50 μL 200 mM rac-SO, react for 15 min, take 100 μL in 1 mL ethyl acetate containing (1 mg / mL n-hexanol is internal standard) for extraction, dried over anhydrous magnesium sulfate, and passed through a 0.22 μm organic membrane.
[0025] The above-mentioned samples after organic membrane filtratio...
Embodiment 3
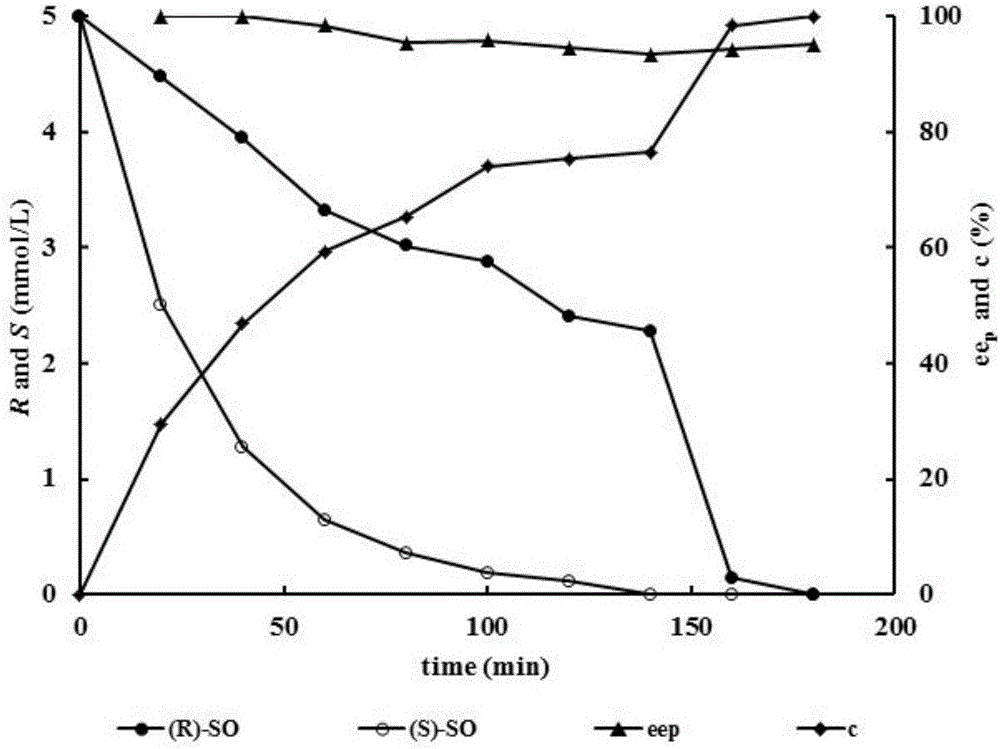
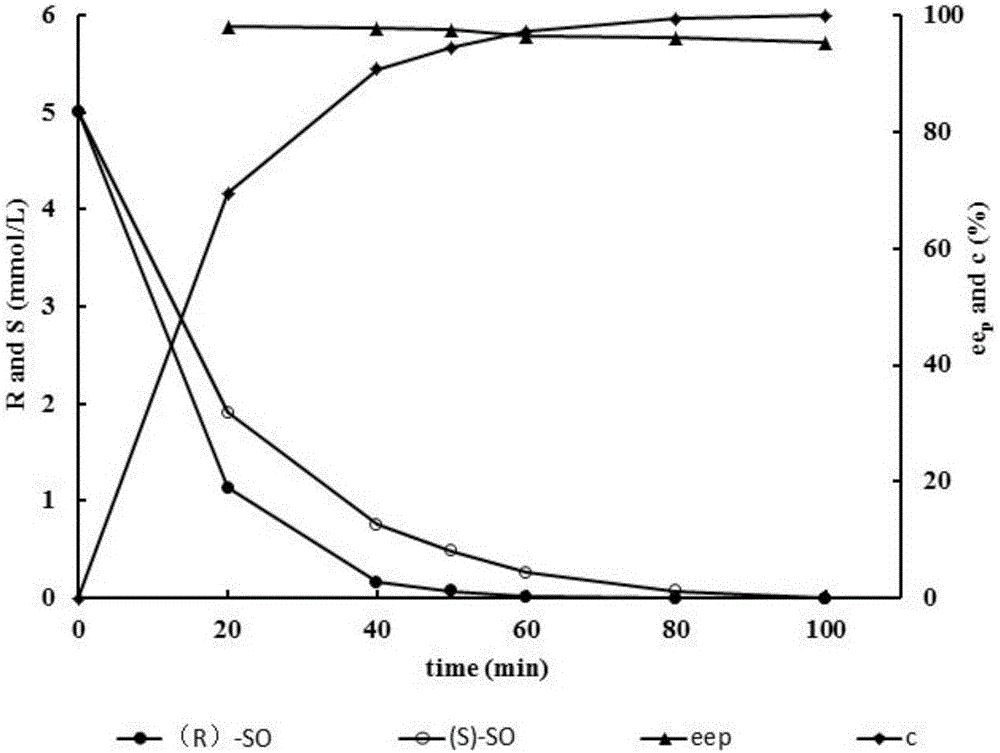
[0026]Embodiment 3 dual enzymes add sequence to conversion rate and product ee p Impact
[0027] The two enzymes were added to the catalytic system in sequence: in a 1mL reaction system, 400μl of VrEH3 whole-cell bacterial suspension was added to 517μl of 100mmol / L, pH 7.0 phosphate buffer, preheated at 25°C for 5min, and then added 50μl of rac-SO (final concentration was 10mmol / L L) Start the reaction, take regular samples for GC detection until the hydrolysis of (S)-SO is complete, then add 33 μl AuEH2 A250I Whole-cell bacterial suspensions were used to catalyze the remaining conformational substrate (R)-SO.
[0028] Two enzymes are added to the catalytic system at the same time: in a 1mL reaction system, 400μl VrEH3 and 33μl AuEH2 A250I The whole cell suspension was added to 517 μl of 100 mmol / L, pH 7.0 phosphate buffer, preheated at 25° C. for 5 min, and 50 μl of rac-SO (final concentration 10 mmol / L) was added to start the reaction. During the reaction process, samples...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com