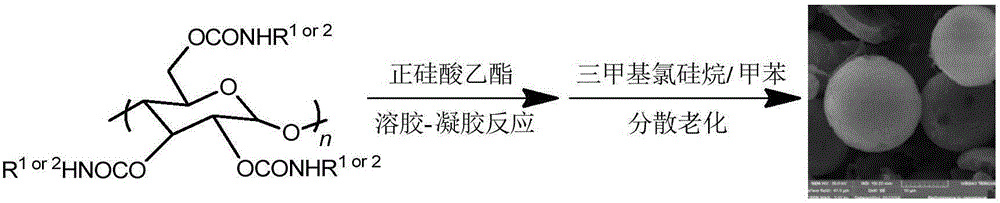
Preparation method of functional inorganic silicon-based chiral microsphere fixed-phase material based on amylose derivatives
An amylose and functionalization technology, applied in the field of preparation of chiral microsphere stationary phase materials, can solve the problems of reduced separation efficiency, tailing, low yield, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0017] 1. Take 0.2g of microcrystalline amylose and vacuum-dry at high temperature for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 12h; add lithium chloride after cooling to room temperature (the mass is the mass of amylose After stirring for 3 hours, heat up again, add anhydrous pyridine, add an appropriate amount of 3,5-dimethylphenyl isocyanate (77mol% of the amylose hydroxyl group) after 2 hours of reflux, continue stirring and reflux for 8 hours, and then add a small amount of 3-(trimethoxysilyl) propyl isocyanate (3.1mol% of amylose hydroxyl group), continued to stir and reflux for 12h and then treated with excess 3,5-dimethylphenylisocyanate (90mol% of amylose hydroxyl group ), continue to stir and reflux for 8 hours, then stop the reaction; cool to room temperature, add methanol to settle, filter and wash, and vacuum-dry at 60°C to constant weight, with a yield of 87%. The introduction ratio of 3,5-dimethylphenyl carbamate to 3-(trimethoxysilyl)prop...
specific Embodiment approach 2
[0022] 1. Take 0.2g of microcrystalline amylose and vacuum-dry at high temperature for 4h, then stir and reflux in anhydrous N,N-dimethylacetamide for 12h; add lithium chloride after cooling to room temperature (the mass is the mass of amylose After stirring for 3 hours, heat up again, add anhydrous pyridine, add an appropriate amount of 3,5-dimethylphenyl isocyanate (77mol% of the amylose hydroxyl group) after 2 hours of reflux, continue stirring and reflux for 8 hours, and then add a small amount of 3-(trimethoxysilyl)propyl isocyanate (6.9mol% of amylose hydroxyl group) was treated with excess 3,5-dimethylphenylisocyanate (90mol% of amylose hydroxyl group) after continuous stirring and reflux for 12h , continue to stir and reflux for 8h to stop the reaction; cool to room temperature, add methanol to settle, filter and wash, vacuum dry at 60°C to constant weight, and the yield is 85%. The introduction ratio of 3,5-dimethylphenyl carbamate to 3-(trimethoxysilyl)propyl carbama...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com