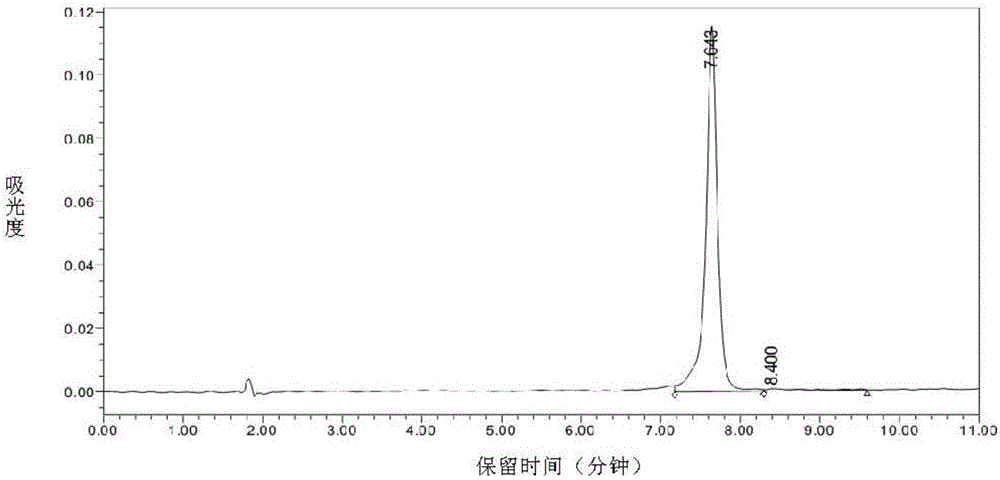
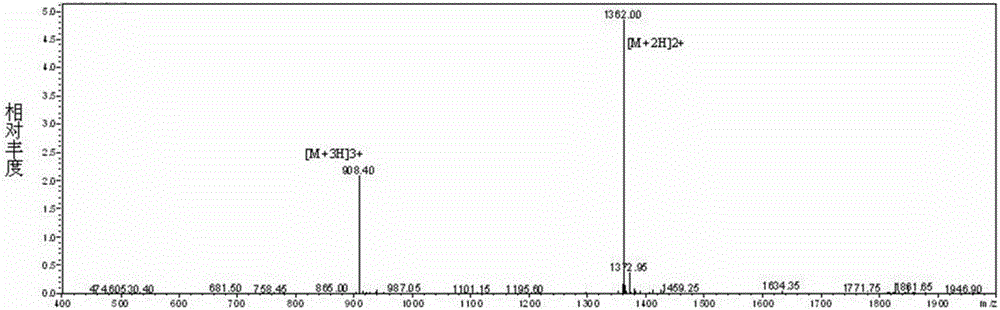
Immunogen HM4-M2e as well as preparation method and application thereof
A sequence and protein technology, applied in biochemical equipment and methods, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of poor immunogenicity and small molecular weight, achieve high cross-linking rate, short preparation time, and reduce production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1, the design and synthesis of polypeptide M2A6
[0087] 1. Design of polypeptide M2A6
[0088] The present invention designs a polypeptide M2e by comparing the sequences of about 13,000 influenza strains from NCBI, the amino acid sequence of which is shown in Sequence 1, and names it as polypeptide M2A6.
[0089] 2. Synthesis of polypeptide M2A6
[0090] The present invention adopts polypeptide solid-phase synthesis method to synthesize polypeptide M2A6. Based on Fmoc chemical synthesis, the carboxyl group of the C-terminal amino acid of the target polypeptide to be synthesized is first connected with an insoluble polymer resin in the form of a covalent bond, and then the amino acid is As the starting point of peptide synthesis, the amino group of the amino acid reacts with the activated carboxyl group of other amino acids to form a peptide bond. This process is repeated continuously, and the peptide can be obtained by synthesizing from the C-terminus to th...
Embodiment 2
[0105] Embodiment 2, the preparation of immunogen HM4-M2A6
[0106] One, the preparation method of immunogen HM4-M2e
[0107] 1. Solution preparation
[0108] (1) Preparation of carrier protein solution: with HM4 protein (the amino acid sequence of HM4 protein is shown in sequence 2 in the sequence listing) as carrier protein, PBS buffer (20mM phosphate, 150mM sodium chloride, pH7.4) as solvent , preparing a carrier protein solution to obtain a carrier protein solution with a concentration of 1.5 mg / ml;
[0109] (2) Preparation of SMCC solution: According to 30× excess calculation, weigh 3.3 mg of heterobifunctional cross-linking agent SMCC and dissolve it in 165 μL of DMF solution to obtain a SMCC solution with a concentration of 20 mg / mL;
[0110] (3) Preparation of target peptide solution: Calculated at 1×, weigh 0.45 mg of the polypeptide M2A6 prepared in Example 1 above and dissolve it in 150 μL of PBS buffer to obtain a target peptide solution with a concentration of 3...
Embodiment 3
[0119] Example 3, Preparation of HM4-M2A6 conjugate vaccine and its immune effect
[0120] 1. Preparation of HM4-M2A6 conjugate vaccine
[0121] Dilute the immunogen HM4-M2A6 prepared in step 1 with PBS buffer (20 mM phosphate, 150 mM sodium chloride, pH7.4) to a concentration of 250 μg / mL, and then slowly add aluminum hydroxide adjuvant, The final concentration of the aluminum hydroxide adjuvant was 2.5 mg / mL, and the sample was slowly shaken during the dropwise addition. After adding aluminum hydroxide, seal the sample well, place it at 2-8°C for 16 hours with slow shaking and absorb it, then dilute it to a final antigen concentration of 100 μg / mL to obtain the HM4-M2A6 conjugate vaccine.
[0122] Second, the detection of antibody titer
[0123] (1) Immunization method
[0124] Inject the HM4-M2A6 conjugate vaccine prepared in step 1 by intraperitoneal injection. The immunization dose is 50μg / 0.5mL / bird / time. The experimental animal information is shown in Table 1.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com