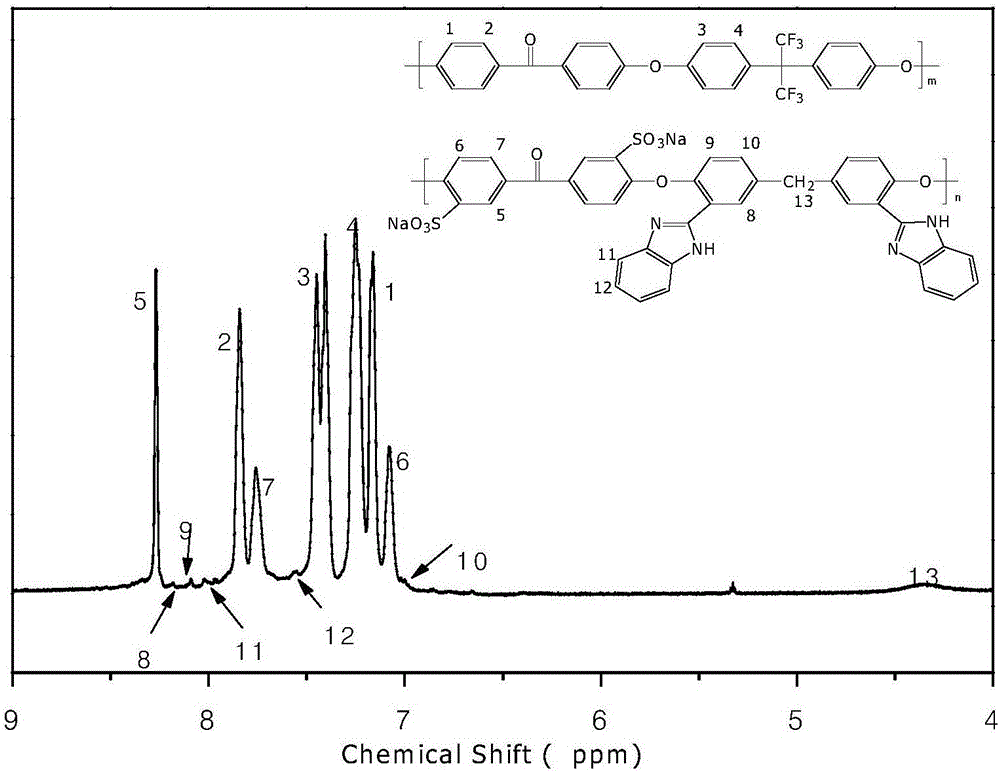
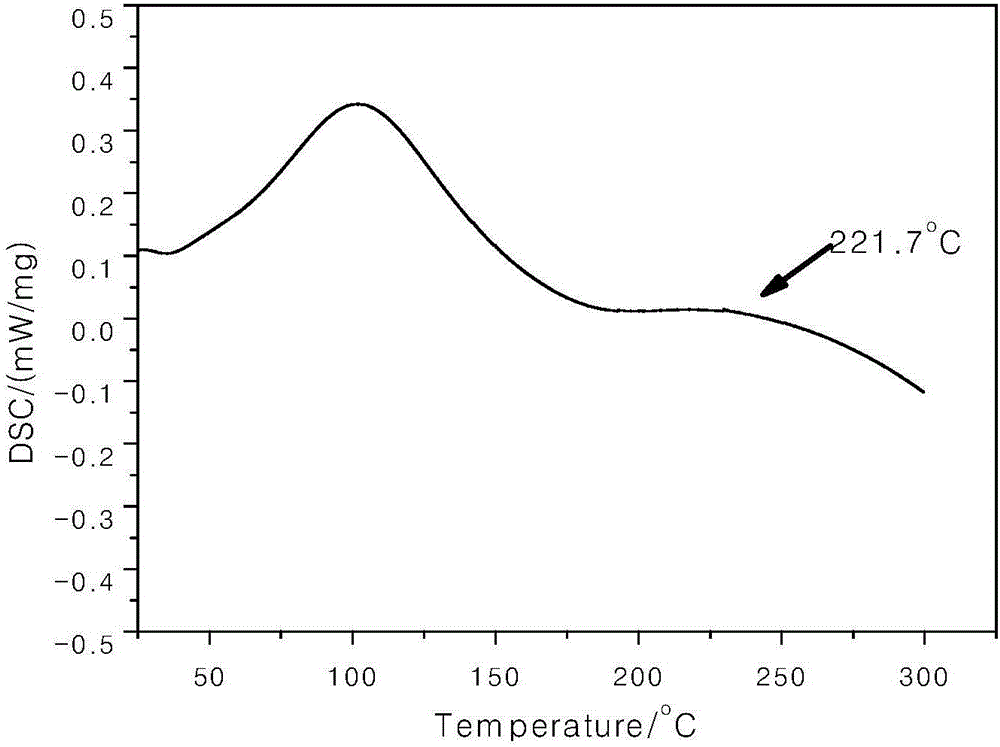
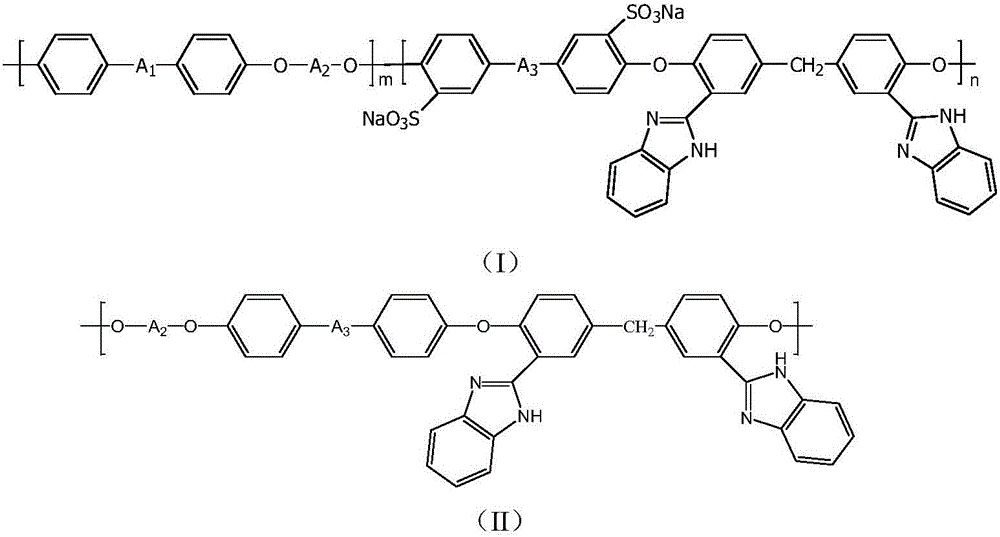
Polyaryletherketone/polyether sulphone with side chain containing benzimidazole and preparation method and application of polyaryletherketone/polyether sulphone
A technology of benzimidazole and polyaryl ether ketone, which is applied in the field of polymer materials and their preparation, can solve the problems of shortening the lifespan of membrane materials, reducing the anti-oxidation stability, reducing mechanical properties, etc., so as to simplify the synthesis steps and improve the comprehensive performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Add 4,4'-difluorobenzophenone (0.4364g, 2mmol) shown in formula (i) into a 100mL three-necked flask equipped with a magnetic stirrer, a nitrogen pipe, a water separator and a condenser tube, the formula ( 3,3'-sodium disulfonate-4,4'-difluorobenzophenone (1.2669g, 3mmol) shown in ii), hexafluorobisphenol A (1.5130g, 4.5mmol) shown in formula (iii) ), 4,4'-methylene-2,2'-benzimidazole bisphenol (0.2162g, 0.5mmol) and potassium carbonate (0.8293g, 6mmol) shown in formula (iv), with 17mL N,N - Dissolve dimethylacetamide, add 15mL of toluene as a water-carrying agent, pass nitrogen to remove oxygen for 10 minutes, heat up to 145-150°C and reflux for 4 hours, distill the toluene, then raise the temperature to 160-165°C to continue the reaction for 20 hours (the solution viscosity is obvious increase), stop the reaction. After the reaction liquid was cooled, the reaction liquid was diluted with 5 mL of N,N-dimethylacetamide, poured into 200 mL of ethanol, precipitated, and fi...
Embodiment 2
[0054] Add 4,4'-difluorodiphenylsulfone (0.7628g, 3mmol) shown in formula (v) into a 100mL three-necked flask equipped with a magnetic stirrer, nitrogen pipe, water separator and condenser tube, the formula ( ii) 3,3'-sodium disulfonate-4,4'-difluorobenzophenone (0.8446g, 2mmol), hexafluorobisphenol A (1.3449g, 4mmol) represented by formula (iii) , 4,4'-methylene-2,2'-benzimidazole bisphenol (0.4325g, 1mmol) and potassium carbonate (0.8293g, 6mmol) represented by formula (iv), with 17mL N,N-di Dissolve methyl acetamide, add 15mL toluene as a water-carrying agent, pass nitrogen to remove oxygen for 10 minutes, heat up to 145-150°C and reflux for 4 hours, distill the toluene, then raise the temperature to 160-165°C to continue the reaction for 22 hours (the viscosity of the solution increases significantly ), stop the reaction. After the reaction liquid was cooled, the reaction liquid was diluted with 5 mL of N,N-dimethylacetamide, poured into 200 mL of ethanol, precipitated, a...
Embodiment 3
[0059] Add 4,4'-difluorodiphenyl sulfone (0.2542g, 1mmol) shown in formula (v) into a 100mL three-necked flask equipped with a magnetic stirrer, nitrogen pipe, water separator and condenser tube, the formula ( ii) 3,3'-sodium disulfonate-4,4'-difluorobenzophenone (1.6892g, 4mmol), bisphenol A (0.6849g, 3mmol) shown in formula (vi), formula 4,4'-methylene-2,2'-benzimidazole bisphenol (0.8649g, 2mmol) and potassium carbonate (0.8293g, 6mmol) shown in (iv), with 17mL N,N-dimethyl Dissolve acetamide, add 15mL of toluene as a water-carrying agent, pass nitrogen to remove oxygen for 10 minutes, raise the temperature to 145-150°C and reflux for 4 hours, distill the toluene, then raise the temperature to 160-165°C to continue the reaction for 22 hours (the viscosity of the solution increases significantly), Stop responding. After the reaction liquid was cooled, the reaction liquid was diluted with 5 mL of N,N-dimethylacetamide, poured into 200 mL of ethanol, precipitated, and filtere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com