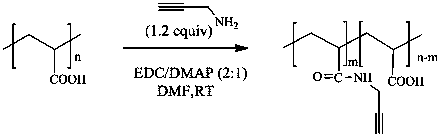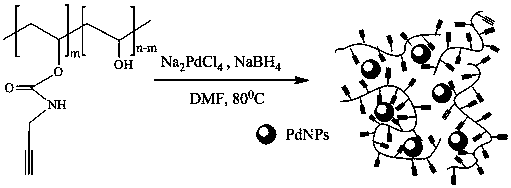Preparation method of composite hydrogen-absorbing material supported by alkyne-rich polymer supported nano-palladium catalyst and hydrogen-absorbing material
A polymer-loaded, hydrogen-absorbing material technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of reduced hydrogen elimination performance and difficulty in fully contacting and other issues to achieve the effect of improving hydrogen absorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
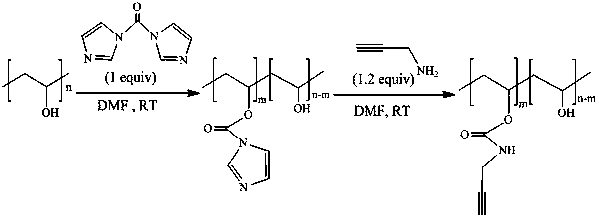
[0051] Example 1 Preparation of Alkyne Functionalized Polyvinyl Alcohol
[0052] Under the protection of nitrogen, 20 mL DMF and 1.0648 g PVA (20 mmol) were added into a three-necked flask, stirred in an oil bath at 85 °C until the PVA was completely dissolved, cooled to room temperature, and placed under N 2 Under protection, add 3.243 g CDI (20 mmol) and react at room temperature for 3 h; then, add 1.54 mL propargylamine (24 mmol) to the three-necked flask and react at room temperature for 20 h; then slowly pour the reaction solution in the three-necked flask into a container Stir in a beaker with 100 mL of ethanol solution until all the product is precipitated, repeatedly wash with ethanol solution, filter, and vacuum-dry the filter residue for 24 h to constant weight to obtain a white flocculent solid.
[0053] The results of structural confirmation are as follows: 1 H NMR (400 MHz, DMSO) δ 4.73 (m, 1H), 4.4-4.37 (m, 1H), 3.75 (s, 2H), 3.03 (s, 1H), 1.97-1.93 (m, 3H), 1.7...
Embodiment 2
[0054] Example 2 Preparation of Alkyne Functionalized Polyacrylic Acid
[0055] Add 1.44 g of PAA (20 mmol) into a three-necked flask containing 20 mL of DMF, stir for 2 h, heat until PAA is completely dissolved, and cool to room temperature; then, add 4.6 g of EDCI and 1.46 g of DMAP to the three-necked flask, After stirring for a period of time, add 1.54 mL of propargylamine (24 mmol), and after stirring for 20 h, stop the reaction to obtain a reaction solution; concentrate the prepared reaction solution to about 8 mL, pour it into a solution containing 100 mL of dichloromethane , stirred until all the products were precipitated, washed repeatedly with acetone and dichloromethane solutions, filtered, and the filter residue was vacuum-dried for 24 h to constant weight to obtain a yellow solid.
[0056] The results of structural confirmation are as follows: 1 H NMR (300 MHz, DMSO) δ3.31(s, 2H), 2.99(s, 1H), 2.56(s, 1H), 1.74-1.77(m, 2H); 13 C NMR (300 MHz, DMSO) δ169.82, 79....
Embodiment 3
[0057] Example 3 Preparation of Alkyne-rich Polyvinyl Alcohol-supported Nano Palladium Catalyst Composite Hydrogen Absorbing Material
[0058] Weigh 0.5 g of alkyne-functionalized polyvinyl alcohol product and dissolve it in 50 mL of DMF solution. After fully dissolving, add 0.0651 g (0.2212 mmoL) of sodium tetrachloropalladate aqueous solution (5 mL) and stir for 3 min; then While stirring at a high speed, a fresh sodium borohydride (0.4425 mmol) aqueous solution (0.5 mL) was slowly added dropwise and stirred vigorously for 5 min to obtain a tan alkyne-rich PVA-PdNPs suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com