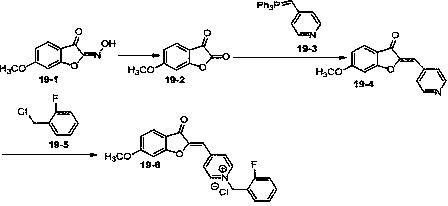
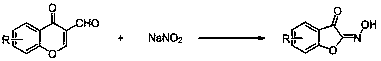A kind of preparation method and application of benzofuran-2,3-diketoxime derivative
A technology for benzofuran and derivatives, which is applied in the field of preparation of organic compounds, and can solve the problem of high equipment corrosion, great influence on operators, and difficulties in the source of raw materials for the synthesis route of benzofuran-2,3-diketoxime derivatives and other problems, to achieve the effects of short reaction time, low cost, and many types of product structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 2-(hydroxyimino)-benzofuran-3(2H)-one
[0027] With 4-oxo-4H-benzopyran-3-carbaldehyde and sodium nitrite as raw materials, the reaction steps are as follows:
[0028] Add 4-oxo-4H-chromene-3-carbaldehyde (0.174 g, 1.0 mmol), sodium nitrite (0.069 g, 1.0 mmol), potassium persulfate (0.270 g, 1.0 mmol) and Acetone (4 ml), react at room temperature;
[0029] TLC tracking reaction until complete completion;
[0030] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 61%). The analytical data of the product are as follows: 1 H NMR (400 MHz, DMSO- d 6 ): δ 12.45 (s, 1H),7.82 (t, J = 7.4 Hz, 1H), 7.75 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 8.3 Hz, 1H), 7.33 (t, J = 7.5 Hz, 1H).
Embodiment 2
[0031] Example 2: Synthesis of 2-(hydroxyimino)-5-fluoro-benzofuran-3(2H)-one
[0032] Using 4-oxo-4H-6-fluorobenzopyran-3-carbaldehyde and sodium nitrite as raw materials, the reaction steps are as follows:
[0033] Add 4-oxo-4H-6-fluoro-benzopyran-3-carbaldehyde (0.192 g, 1.0 mmol), sodium nitrite (0.138 g, 2.0 mmol), potassium persulfate (0.59 g, 2.0 mmol) and acetone (4 ml), react at room temperature;
[0034] TLC tracking reaction until complete completion;
[0035] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 83%). The analytical data of the product are as follows: 1 H NMR (400 MHz, DMSO- d 6 ): δ 12.52 (s, 1H), 7.68 (td, J = 9.0, 2.8 Hz, 1H), 7.58 (dd, J = 6.9, 2.8 Hz, 1H), 7.52 (dd, J=9.0, 3.7 Hz, 1H).
Embodiment 3
[0036] Example 3: Synthesis of 2-(hydroxyimino)-5-chloro-benzofuran-3(2H)-one
[0037] With 4-oxo-4H-6-chloro-benzopyran-3-carbaldehyde and sodium nitrite as raw materials, the reaction steps are as follows:
[0038] Add 4-oxo-4H-6-chloro-chromene-3-carbaldehyde (0.209 g, 1.0 mmol), sodium nitrite (0.207 g, 3.0 mmol), potassium persulfate (0.81 g, 3.0 mmol) and acetone (4 ml), react at room temperature;
[0039] TLC tracking reaction until complete completion;
[0040] The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 86%). The analytical data of the product are as follows: 1 H NMR (400 MHz, DMSO- d 6 ): δ 12.60 (s, 1H), 7.82 (dd, J = 8.8, 2.4 Hz, 1H), 7.76 (d, J = 2.2 Hz, 1H), 7.51 (d, J = 8.8Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com