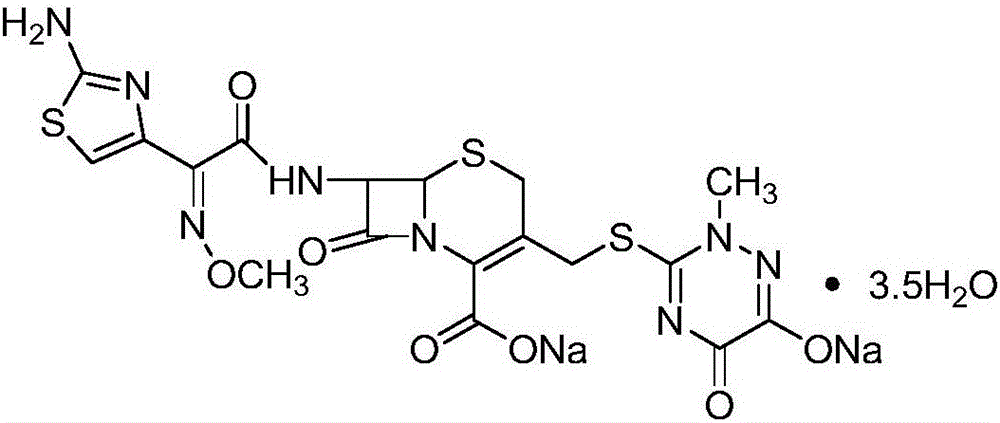
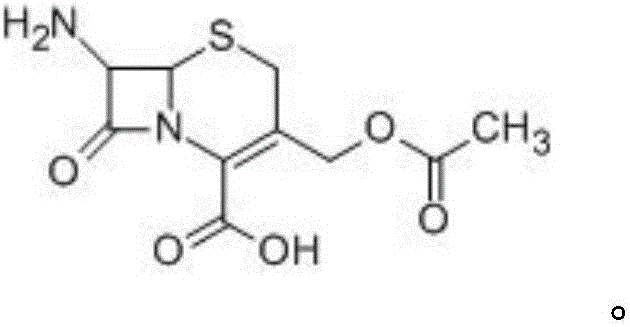
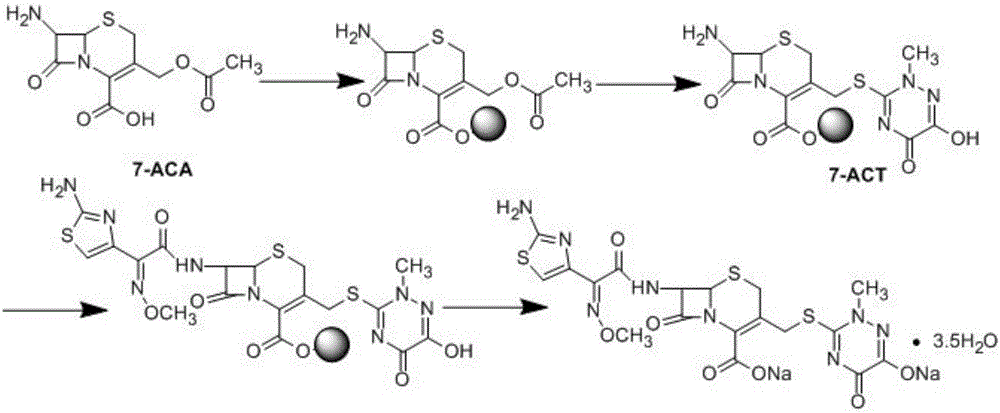
Solid-phase synthesizing method of ceftriaxone sodium
A technology of ceftriaxone sodium and solid-phase synthesis, which is applied in the field of chemical pharmacy, can solve the problems of low total yield, cumbersome operation, and poor purity, and achieve the effects of improving production efficiency, simplifying reaction procedures, and reducing losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Bridging of 7-ACA and solid phase carrier
[0066]
[0067] In the glazed glass column, react 25g 7-ACA and 100g chloromethyl resin in 100ml DMF, TLC detects that there is no raw material residue, so that it is fully bridged with the solid phase carrier, and after the reaction is completed, use N 2 (Gas such as argon, air, helium) stream to remove solvent, then wash the resin successively with 100ml of dichloromethane and pyridine (or chloroform, toluene and other solvents) respectively, and directly carry out the next step reaction.
[0068] In Embodiment 1, the chloromethyl resin can also be replaced by polystyrene-styrene divinyl cross-linked resin, polyacrylamide, polyethylene-glycol resin, carboxyl resin, amino resin, hydrazide resin, and the like.
[0069] According to the functional group difference of the resin, the dosage ratio of 7-ACA and resin was adjusted. For example: when polystyrene and polyethylene resins are used, the dosage of 7-ACA is ...
Embodiment 2
[0072] Embodiment two: prepare the ceftriaxone of solid phase bridging
[0073]
[0074] A. In the glazed glass column, add 30g of 6-hydroxy-3-mercapto-2-methyl-1,2,4-triazin-5-one in acetonitrile (just completely dissolved state) solution, and heat under nitrogen protection To 50°C, add 56g of catalyst boron trifluoride and react for 2h under stirring. After the reaction is completed, use N 2 (Gas such as argon, air, helium) stream to remove solvent, then wash the resin successively with 100ml of dichloromethane and pyridine (or chloroform, toluene and other solvents) respectively, and directly carry out the next step reaction.
[0075] In this step, 6-hydroxy-3-mercapto-2-methyl-1,2,4-triazin-5-one can also be replaced by a triazine compound with other sulfur-containing leaving groups at the 3-position ,like:
[0076] X: It is a leaving group with structures such as chlorine, iodine, bromine, ester group, and acid halide group.
[0077] The amount of the triazine com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com