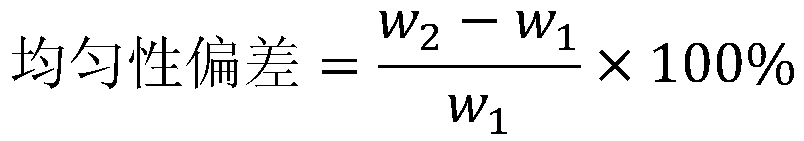Coating Process of Drug Coatings on Medical Devices
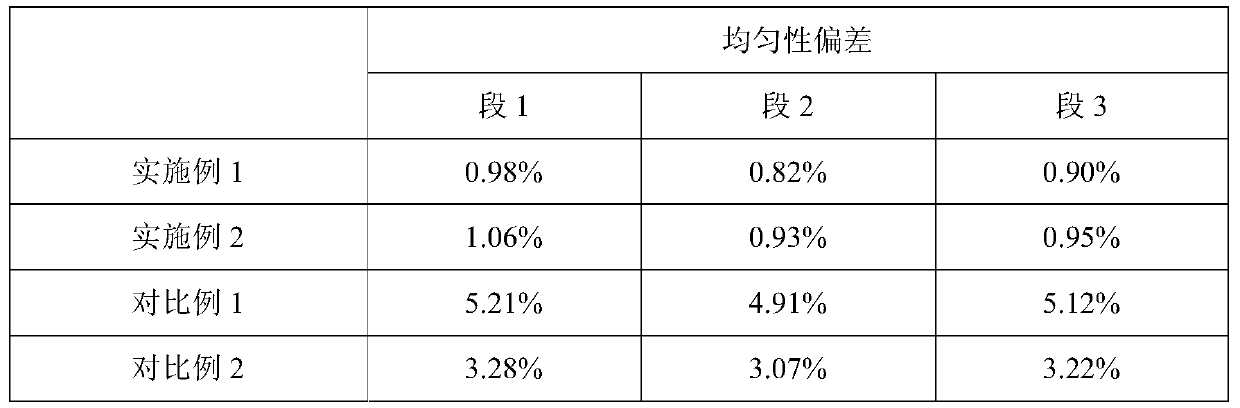
A medical device and drug coating technology, applied in the field of medical devices, can solve the problems of insufficient bonding force between the drug and the surface of the medical device, insufficient crystal transformation of the drug coating, and insufficient uniformity of drug particle size, etc., so as to improve the release curve, The effect of uniform size and consistent crystal state
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Coating process of paclitaxel coating on drug-eluting stent
[0027] 1) Preparation of the spraying solution: Weigh about 0.15g of paclitaxel and add it into a 25ml glass bottle; add 10ml of ethanol into the glass bottle; keep warm in an oven at 45°C until the paclitaxel is completely dissolved.
[0028] 2) Stent coating: place the stent under the ultrasonic spray head, set the ultrasonic frequency to 30khz, the flow rate of the spray solution to 0.05ml / min, and the flow rate of the carrier gas (nitrogen gas) to 2L / min. After spraying, remove the bracket.
[0029] 3) The scaffold is dried at room temperature for 30 minutes.
[0030] 4) Scaffold solvent treatment: place an acetonitrile aqueous solution with a volume fraction of 25% on the lower layer of the container, place the sprayed stent on the upper layer of the container, seal the container and place it in an oven at 25° C., evaporate the acetonitrile aqueous solution to form a steam-filled container, ...
Embodiment 2
[0031] Example 2: Coating process of rapamycin coating on drug-eluting stent
[0032] 1) Preparation of the spraying solution: Weigh about 0.17g of rapamycin and add it into a 25ml glass bottle; add 10ml of ethanol into the glass bottle; keep warm in an oven at 45°C until the rapamycin is completely dissolved.
[0033] 2) Stent coating: place the stent under the ultrasonic spray head, set the ultrasonic frequency to 30khz, the flow rate of the spray solution to 0.05ml / min, and the flow rate of the carrier gas (air) to 2L / min. After spraying, remove the bracket.
[0034] 3) The scaffold is dried at room temperature for 30 minutes.
[0035] 4) Scaffold solvent treatment: place an acetonitrile aqueous solution with a volume fraction of 25% on the lower layer of the container, place the sprayed stent on the upper layer of the container, seal the container and place it in an oven at 20° C., evaporate the acetonitrile aqueous solution to form a steam-filled container, The stent wa...
Embodiment 3
[0036] Embodiment 3: the coating process of paclitaxel coating on drug-coated balloon
[0037] 1) Preparation of the spraying solution: Weigh about 0.15g of paclitaxel and add it into a 25ml glass bottle; add 10ml of ethanol into the glass bottle; keep warm in an oven at 45°C until the paclitaxel is completely dissolved.
[0038] 2) Balloon coating: place the balloon under the ultrasonic spray head, set the ultrasonic frequency to 30khz, the flow rate of the spray solution to 0.05ml / min, and the flow rate of carrier gas (nitrogen) to 2L / min. After spraying, remove the balloon.
[0039] 3) The balloon is dried at room temperature for 30 minutes.
[0040] 4) Balloon solvent treatment: place an acetonitrile aqueous solution with a volume fraction of 25% on the lower layer of the container, place the sprayed balloon on the upper layer of the container, seal the container and place it in an oven at 30° C., evaporate the acetonitrile aqueous solution to form a vapor filling Containe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com