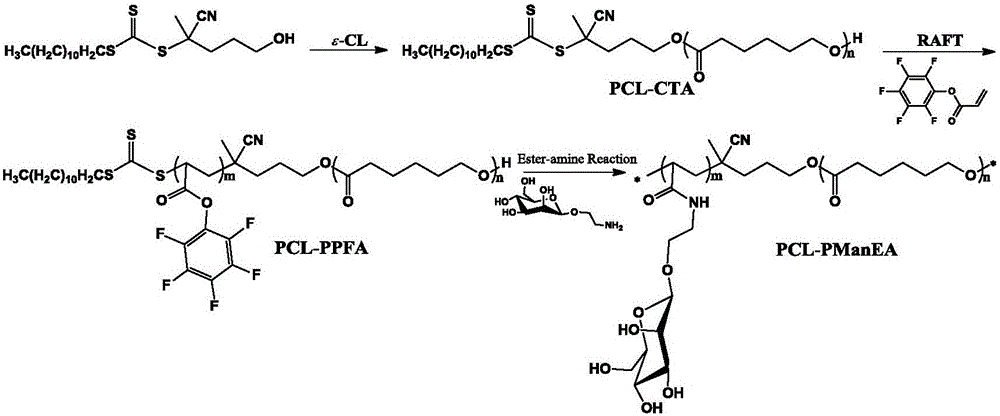
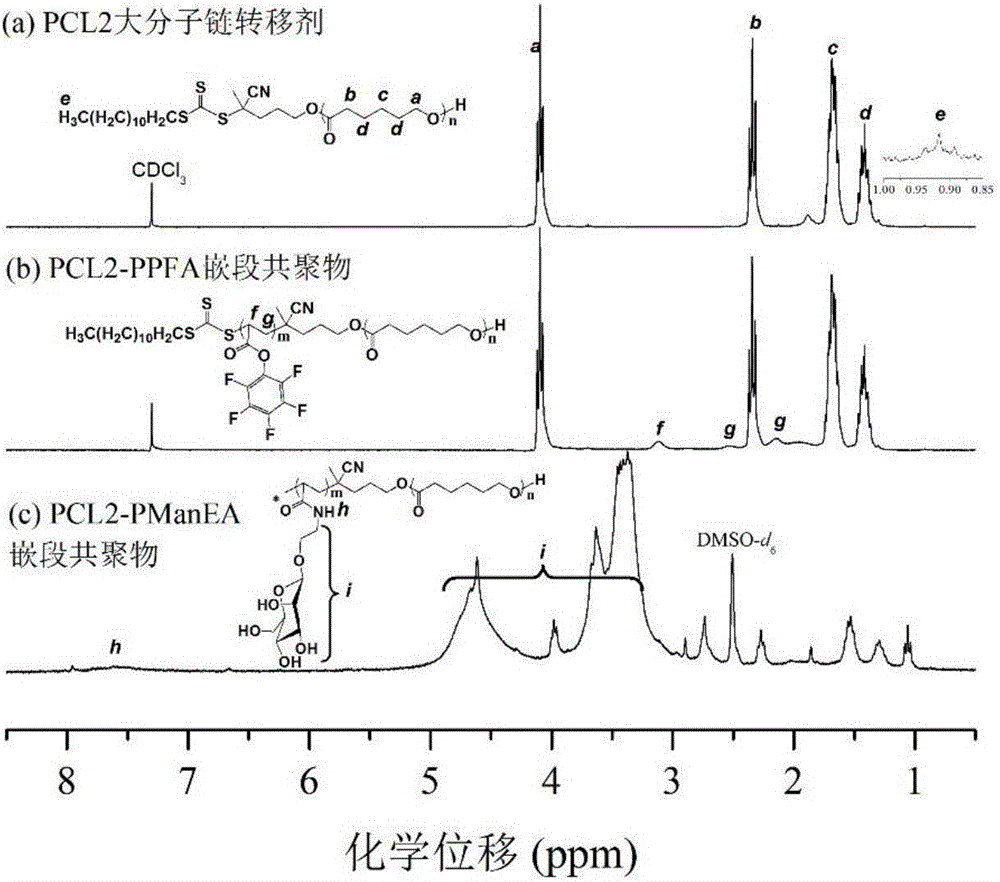
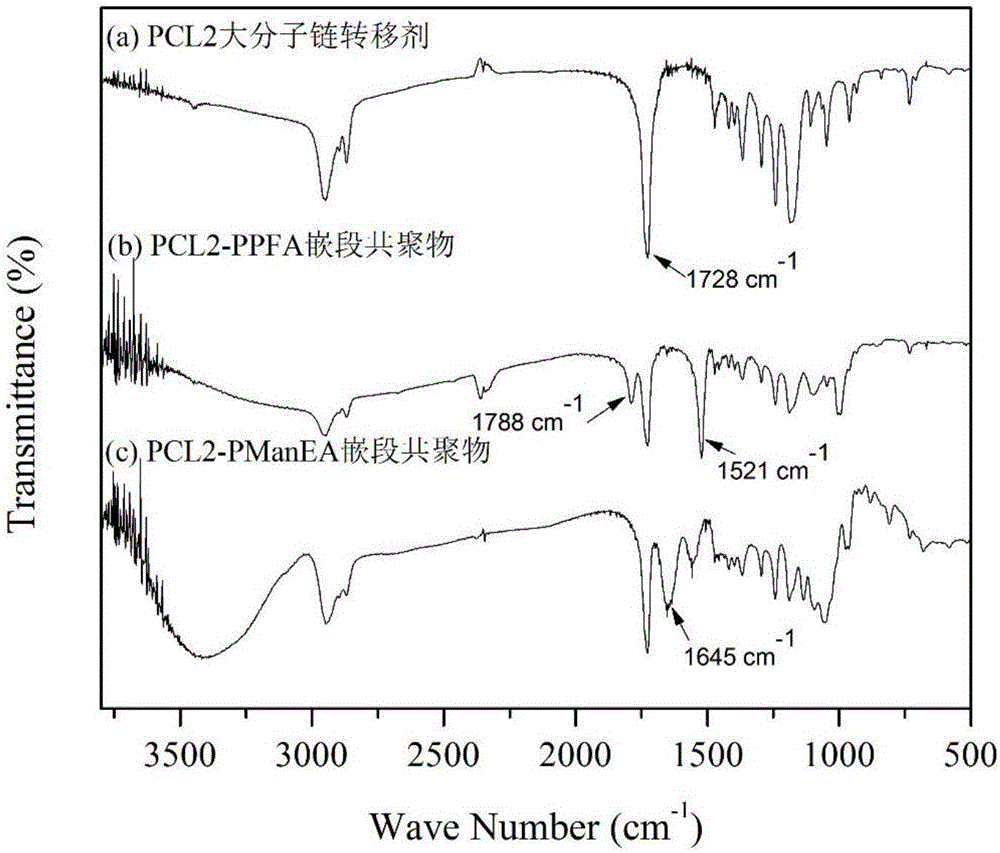
Sugar-containing amphiphilic segmented copolymer and preparation method thereof
A technology of amphiphilic block and copolymer, which is applied in the field of synthesis and preparation of polymer materials, can solve the problems of cumbersome steps of unsaturated sugar-containing monomers, achieve simple polymer grafting means, eliminate synthesis steps and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of PCL1 macromolecular chain transfer agent comprises the following steps:
[0033] 1) Take stannous octoate as a catalyst and 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol as an initiator, add 200 mg of 4-cyano in a glass flask successively -4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol and 5.6 milliliters of ε-caprolactone, the molar ratio of initiator to monomer is 1:100;
[0034] 2) After vacuuming-nitrogen filling-vacuumizing cycle, add 40.5 mg of stannous octoate (dissolved in 2 ml of toluene) catalyst, seal it under nitrogen atmosphere, place it in an oil bath at 110°C for 12 hours, and cool to room temperature to terminate the polymerization;
[0035] 3) adding 10 ml of tetrahydrofuran for dilution, adding the reaction solution dropwise into 200 ml of cold ether solution, filtering and drying to obtain the PCL1 macromolecular chain transfer agent.
Embodiment 2
[0037] The preparation method of PCL2 macromolecular chain transfer agent comprises the following steps:
[0038] 1) Take stannous octoate as a catalyst and 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol as an initiator, add 200 mg of 4-cyano in a glass flask successively -4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol and 8.4 milliliters of ε-caprolactone, the molar ratio of initiator to monomer is 1:150;
[0039] 2) After vacuuming-nitrogen filling-vacuumizing cycle, add 40.5 mg of stannous octoate (dissolved in 2 ml of toluene in advance) catalyst, seal under nitrogen atmosphere, place in 110 ℃ oil bath for 12 hours, cool to room temperature to terminate polymerization;
[0040]3) adding 10 milliliters of tetrahydrofuran for dilution, adding the reaction solution dropwise into 200 milliliters of cold ether solution, filtering and drying to obtain the PCL2 macromolecular chain transfer agent.
Embodiment 3
[0042] The preparation method of PCL1-PPFA block copolymer comprises the following steps:
[0043] 1) Dissolve 1.3 grams of PCL1 macromolecular chain transfer agent in 8 milliliters of toluene solvent, then add 6.2 milligrams of azobisisobutyrocyanide and 1.8 grams of pentafluorophenyl acrylate in turn, and perform a vacuum-filling-vacuum cycle, Seal under a nitrogen atmosphere, place in a 70°C oil bath for 12 hours, and cool to room temperature to terminate polymerization;
[0044] 2) adding 10 ml of tetrahydrofuran for dilution, adding the reaction solution dropwise into 200 ml of cold ethanol solution, filtering and drying to obtain a PCL1-PPFA block copolymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com