drug-coated stent graft
A covered stent and drug application technology, applied in the field of medical devices, can solve the problems of hindering the endothelialization process in the stent cavity, proliferating and restenosis of the stent intima, and increasing the probability of thrombosis, so as to promote the endotheliumization of the lumen and reduce the foreign body. area, reducing the effect of port irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
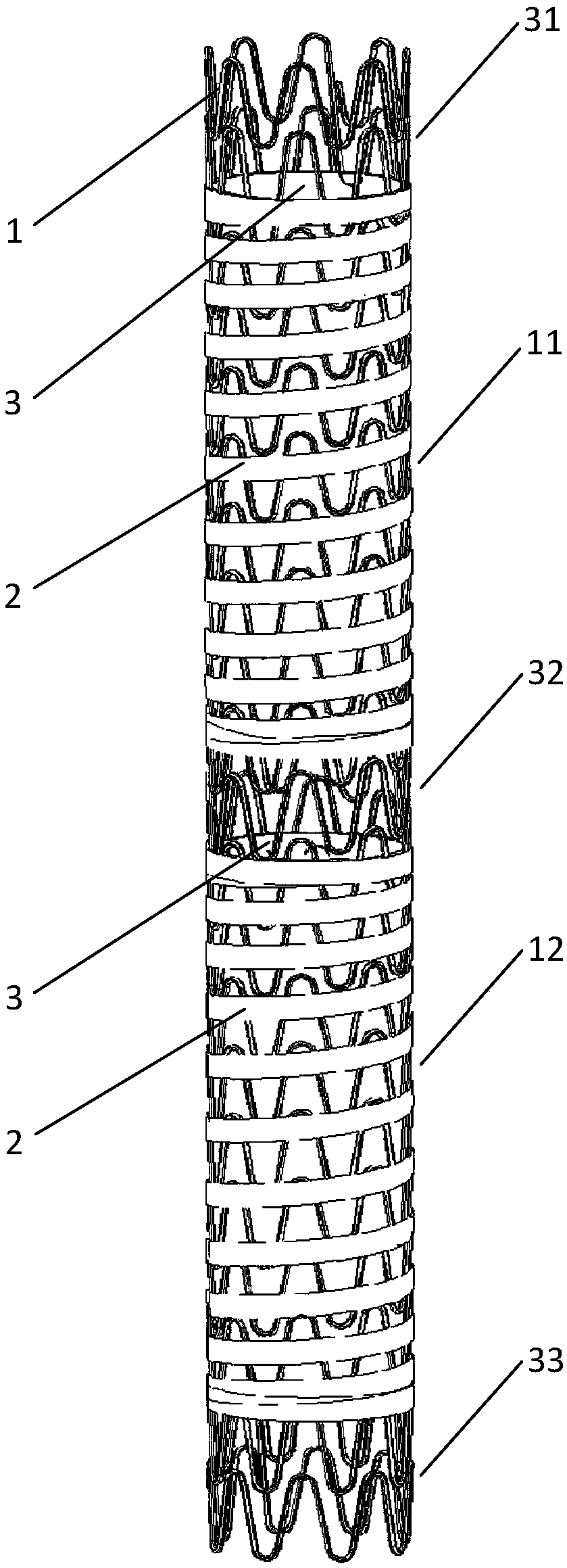
[0044] A drug-coated stent, comprising a stent body 1, an inner covering membrane 2, and an outer membrane 3. The inner covering membrane 2 is closely attached to the inner cavity of the stent body 1, and the inner covering membrane 2 and the stent body 1 fixed connection; the outer membrane 3 is combined with the inner covering membrane 2 by heat sealing, microsurgical suture, etc.; the stent body 1 is provided with an inner covering membrane 2 to form a membrane covering area, and the outer membrane 3 is wound around the The stent body 1 is opposite to the outer surface of the membrane covered area, and the outer membrane 3 firmly wraps the stent body 1 between the inner and outer membranes. On the stent body 1, a bare stent region is formed where the inner membrane 2 is not provided, and the bare stent region and the membrane region are alternately arranged in sequence.
[0045] The stent body 1 is woven from a single wire, the selected single wire has a diameter of 0.1-0.5 mm...
Embodiment 2
[0051] On the basis of Embodiment 1, the metal bracket body is manufactured by laser cutting. Through laser engraving of the pipe, the remaining material and residue are removed, and then through the processes of pickling, electrochemical polishing, cleaning, and drying, a metal bracket body that meets the requirements is formed. Preferably, the tube is a nickel-titanium alloy tube that meets the requirements of the medical implant standard (ASTM F2063), and preferably, the tube has a diameter of 1.65-5.0 mm. In this example, the structure and process of the covered stent covered by the medicine are the same as those in example 1. After testing, the radial support force of the laser-cut stent body before covering is 0.62-1.86N / mm, and the overall radial support force of the coated stent graft is 0.71-1.95N / mm after covering, which improves the stent Support strength to the vascular cavity.
Embodiment 3
[0053] Due to the lumen isolation principle of the coated stents, the endothelialization process of the stent is largely restricted, leading to a high risk of late restenosis. In view of the above shortcomings, under the premise of not affecting the lumen isolation principle of the coated stent graft, a through hole 5 with a certain diameter is added to the inner membrane 2 such as Image 6 As shown, the entrance for the growth of endothelial cells is fully provided, and the process of luminal endothelialization is further accelerated. The method of adding the through hole 5 may be laser drilling. Another way is to retain the bare stent area at both ends, cancel the bare stent area in the middle section of the coated stent graft, and add a large number of through holes with a certain diameter, such as Figure 7 As shown, good results can also be achieved. The above method of adding the through hole 5 may be laser drilling.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com