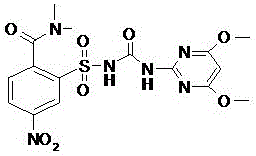
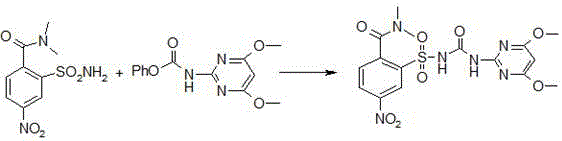
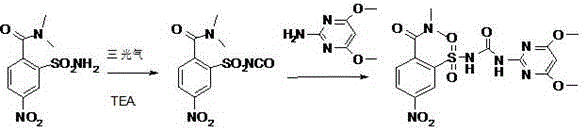
Preparation method of foramsulfuron intermediate of sulfonylurea herbicide
A technology of foramsulfuron and sulfonylureas, applied in the production field of agricultural sulfonylurea herbicides, can solve the problems of inability to produce on a large scale, high cost of raw materials, purification of intermediates, etc., and achieve low cost and high selectivity High efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Example 1: Preparation of methyl N,N-dimethyl-2-sulfonylcarbamate-4-nitrobenzamide (II):
[0041] In a 2000ml four-necked bottle, add 300g of 5-nitro-2-dimethylaminocarbonylbenzenesulfonamide, 1000g of acetone, and 400g of potassium carbonate, start stirring, cool down the ice water to 5°C, and start adding 208g of methyl chloroformate dropwise Soluble in 200g of acetone, after dripping, start to keep warm at 5-10°C until the raw materials basically disappear, and react for about 12 hours; after the reaction is completed, add 1360g of water, stir, control the temperature below 20°C, add dropwise 30% hydrochloric acid to adjust the pH to 2 -3, keep warm for 30 minutes, and start to recover acetone under reduced pressure until it is completely dry. Filter, wash the filter cake with water twice, 300g each time, and dry to obtain light yellow or yellow powdery solid, weight: 349g, yield: 95.8%, content: 99.2%.
[0042] N,N-Dimethyl-2-(N-(N-(4,6-dimethoxypyrimidin-2-yl)-amin...
Embodiment 2
[0046] Example 2: Preparation of N,N-dimethyl-2-sulfonylcarbamate-4-nitrobenzamide (II):
[0047] In a 2000ml four-necked bottle, add 300g of 5-nitro-2-dimethylaminocarbonylbenzenesulfonamide, 1000g of acetone, and 400g of potassium carbonate, start stirring, cool the ice water to 5°C, and start adding 238.8 g of ethyl chloroformate dropwise g dissolved in 200g of acetone, after dropping, start to keep warm at 5-10°C until the raw materials basically disappear, and react for about 12 hours; after the reaction is completed, add 1360g of water, stir, control the temperature below 20°C, and drop 30% hydrochloric acid to adjust the pH to 2 to 3, keep warm for 30 minutes, start to reduce pressure (-0.06 MPa -0.07MPa) to recover acetone until it is completely dry. Filter, wash the filter cake twice with water, 300g each time, and dry to obtain a light brown or yellow powdery solid, weight: 361.6g, yield: 95.2%, content: 99.1%.
[0048] N,N-Dimethyl-2-(N-(N-(4,6-dimethoxypyrimidin-2...
Embodiment 3
[0050] Embodiment 3: preparation of foramsulfuron-methyl;
[0051] Add 1250g of anhydrous formic acid and 400g of compound (I) into a 5L stainless steel autoclave equipped with a hydrogen port, cooling circulating water, pressure gauge and electric heating device, stir and add 25g of 10% palladium carbon to suspend in 375g of anhydrous formic acid Suspension in , sodium molybdate dihydrate 2.5g, then rinse the pipeline with 125g of anhydrous formic acid, close the kettle; feed hydrogen to the internal pressure of 10-15kgf / cm2, and control the temperature at 20-30°C to stir the reaction, during Hydrogen should be added until the pressure no longer drops. The raw material (I) basically disappeared or the content was less than 0.5% under HPLC monitoring; after the reaction was completed, the reaction solution was suction-filtered, and the filter residue was washed twice with formic acid, 100ml each time. Recycling of solid palladium carbon;
[0052] The filtrate was placed in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com