Preparation method of lanthanum hexaboride nanometer powder and application of lanthanum hexaboride nanometer powder
A technology of lanthanum hexaboride and nano-powder, which is applied in the field of ceramic powder preparation, can solve the problems of many impurities, low preparation temperature, and coarse powder particle size, and achieve high purity, good dispersion, and uniform particle size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of lanthanum hexaboride nanopowder of the present invention comprises the following steps:
[0032] (1) According to the La:B molar ratio of 1:6, weigh 1.23g LaCl respectively 3 , 1.14g NaBH 4 , according to the total mass of KCl-LiCl complex salt as LaCl 3 and NaBH 4 5 times the total mass of LiCl, weigh 6.64g KCl and 5.21g LiCl respectively, fully grind and mix the four into the crucible;
[0033] (2) Place the charged crucible in a tube furnace for heat treatment. Under an argon protective atmosphere, raise the temperature to 600°C at a heating rate of 5°C / min, keep it warm for 1h, and then cool down at a rate of 8°C / min To room temperature, the initial product is obtained;
[0034] (3) The initial product was washed several times with deionized water at 60°C until the AgNO 3 When the solution is added dropwise to the filtered washing liquid until there is no white precipitate, it is finally dried to obtain lanthanum hexaboride nanopowder. ...
Embodiment 2
[0037] A preparation method of lanthanum hexaboride nanopowder of the present invention comprises the following steps:
[0038] (1) According to the La:B molar ratio of 1:8, weigh 1.63g La(NO 3 ) 3 , 1.52g NaBH 4 , according to the total mass of KCl-LiCl complex salt as La(NO 3 ) 3 and NaBH 4 10 times the total mass of LiCl, weigh 17.33g KCl and 14.17g LiCl respectively, fully grind and mix them into the crucible;
[0039] (2) Place the charged crucible in a tube furnace for heat treatment. Under an argon protective atmosphere, raise the temperature to 800°C at a heating rate of 5°C / min, keep it warm for 1h, and then cool down at a rate of 5°C / min To room temperature, the initial product is obtained;
[0040] (3) The initial product was washed several times with deionized water at 60°C until the AgNO 3 When the solution is added dropwise to the filtered washing liquid until there is no white precipitate, it is finally dried to obtain lanthanum hexaboride nanopowder.
...
Embodiment 3
[0043] A preparation method of lanthanum hexaboride nanopowder of the present invention comprises the following steps:
[0044] (1) According to the La:B molar ratio of 1:6, weigh 1.23g LaCl respectively 3 , 1.14g NaBH 4 , according to the mass of KCl single-phase salt as LaCl 3 with NaBH 4 5 times the total mass of KCl, take 11.85g KCl, fully grind and mix the three and put them into the crucible;
[0045] (2) Place the charged crucible in a tube furnace for heat treatment. Under an argon protective atmosphere, heat up to 1000°C at a rate of 5°C / min, keep it warm for 2 hours, and then cool down at a rate of 5°C / min To room temperature, the initial product is obtained;
[0046] (3) The initial product was washed several times with deionized water at 60°C until the AgNO 3 When the solution is added dropwise to the filtered washing liquid until there is no white precipitate, it is finally dried to obtain lanthanum hexaboride nanopowder.
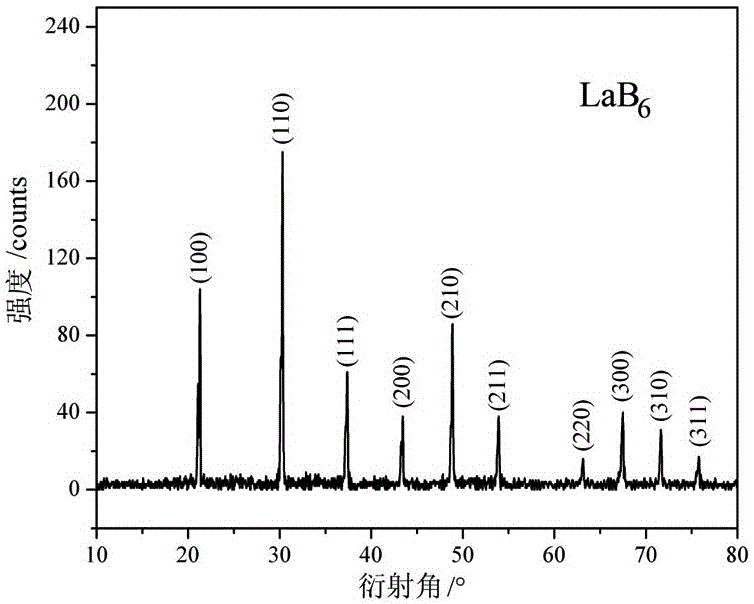
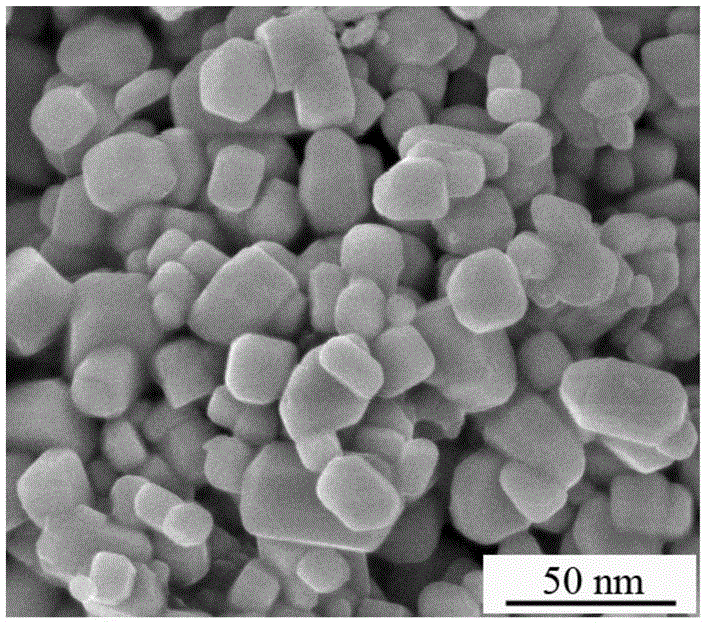
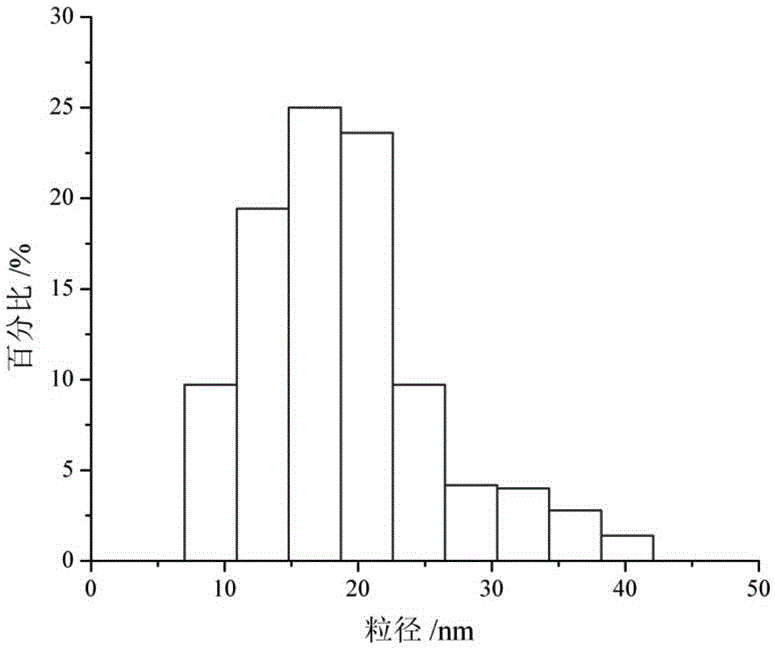
[0047] After detection and analysi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com