10-azaanthracene derivative, and preparation method and applications thereof
A technology of anthracene derivatives and fused heterocycles, which is applied in the field of 10-nitroanthracene derivatives and its preparation, can solve the problems of increasing material cost budget and OLED device manufacturing cost, so as to improve external quantum efficiency and transmittance , cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a preparation method of the 10-aza(hetero)anthracene derivative, including:
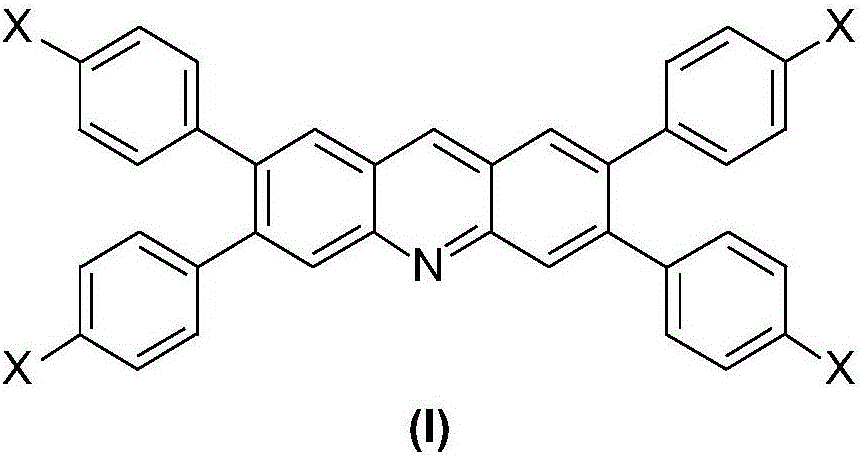
[0046] The compound represented by formula (A) and the compound represented by formula (B) undergo a coupling reaction under the protection of nitrogen to obtain the 10-aza(hetero)anthracene derivative represented by formula (I):
[0047]
[0048] Wherein, X is a substituted or unsubstituted C6-C60 arylamino group or a substituted or unsubstituted C6-C60 fused heterocyclic ring.
[0049] According to the present invention, the intermediate represented by formula (A) is prepared according to the following method:
[0050] (1) reacting 4,5-dibromo-2-nitrobenzaldehyde represented by formula A-1 with 4-bromocyclohexanol represented by C to obtain a compound represented by formula A-2;
[0051] (2) Reacting the compound represented by formula A-2 with elemental bromine to obtain the intermediate represented by formula (A).
[0052]
[0053] In the present invention, the rea...
Embodiment 1
[0061] Example 1: Preparation of Intermediate A
[0062] (1) Synthesis of compound A-2: 154g (0.5mol) of 4,5-dibromo-2-nitrobenzaldehyde (compound A-1), 89g (0.5mol) of 4- Bromocyclohexanol (compound C), 28g (0.05mol) of 1,1'-bis(diphenylphosphine)ferrocene, and 1L of chlorobenzene, then the temperature was raised to 150°C, and the reaction was fully stirred for 24 hours. After the reaction, it was filtered with Celite, washed with toluene and hexane, concentrated, and purified by column chromatography to obtain 73 g of compound A-2 with a yield of 35%. Mass spectrum m / z: 415.96 (calculated value: 415.91). Theoretical element content (%) C 13 H 6 Br 3 N: C, 37.54; H, 1.45; Br, 57.64; N, 3.37. Measured element content (%): C, 37.45; H, 1.37; Br, 57.58; N, 3.15. The above results confirm that the obtained product is the target product.
[0063] (2) Synthesis of Intermediate A: Add 111g (0.83mol) of anhydrous aluminum trichloride to a three-necked flask, add 166g (0.4mol) of compo...
Embodiment 2
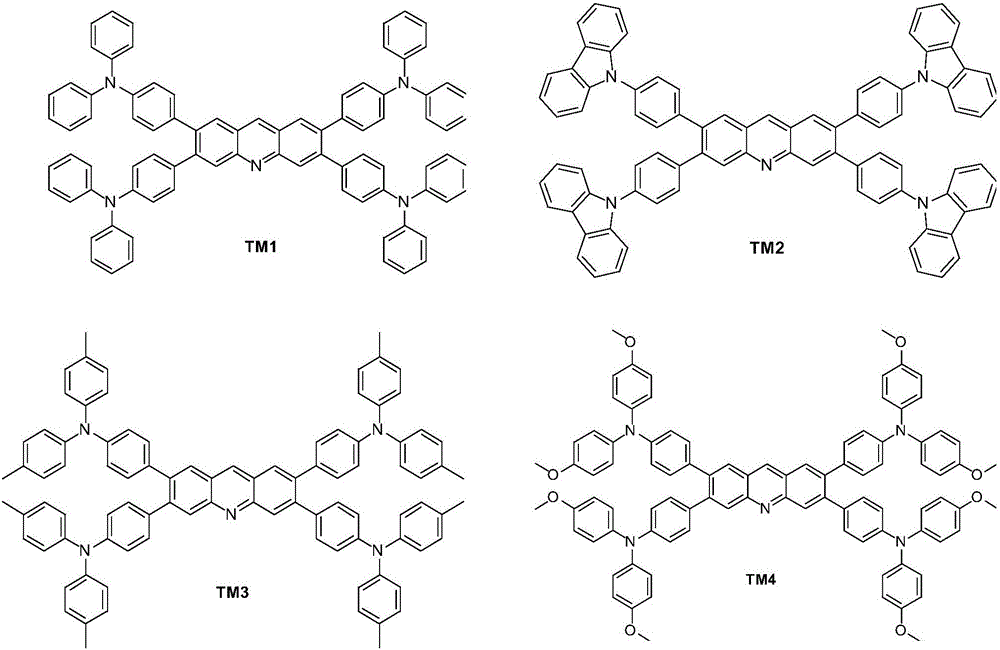
[0064] Example 2: Synthesis of compound TM1
[0065] Add 494mg (1mmol) of Intermediate A and 231mg (0.2mmol) of tetrakis(triphenylphosphine) palladium mixture to 10ml of toluene with removal of air, 2 Under protection, heat the system to 60°C and stir for 5 minutes. 1272 mg (4.4 mmol) of triphenylamine-4-boronic acid, 2.5 ml of de-aired ethanol, and 5 ml of de-aired 2M sodium carbonate aqueous solution were sequentially added to the system, and stirred at 80°C for 24 hours. The reaction system was poured into a large amount of water and extracted with dichloromethane. The organic layer was washed sequentially with saturated brine and water, dried over anhydrous magnesium sulfate, and distilled under reduced pressure. After the remaining solid was purified by column chromatography (hexane / dichloromethane=4:4, V / V), it was recrystallized from a mixed solution of hexane / chloroform to obtain 829 mg (0.72 mmol) of compound TM1 with a yield of 72 %. Mass spectrum m / z: 1152.46 (calcu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com