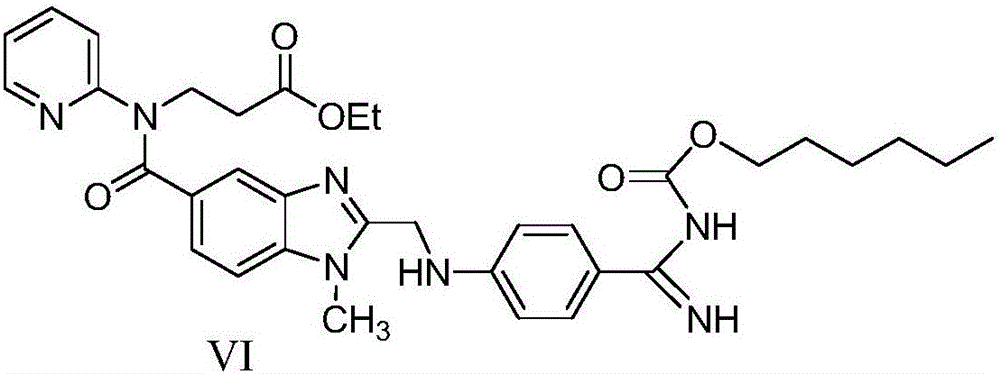
Preparation method for dabigatran etexilate
A technology of dabigatran etexilate and molar ratio, applied in the field of drug synthesis, can solve the problems of easy water absorption and deterioration of CDI, dazzling acid chloride compounds, unfavorable large-scale production, etc., and achieves the effects of low production cost, easy preparation, and simplified production operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
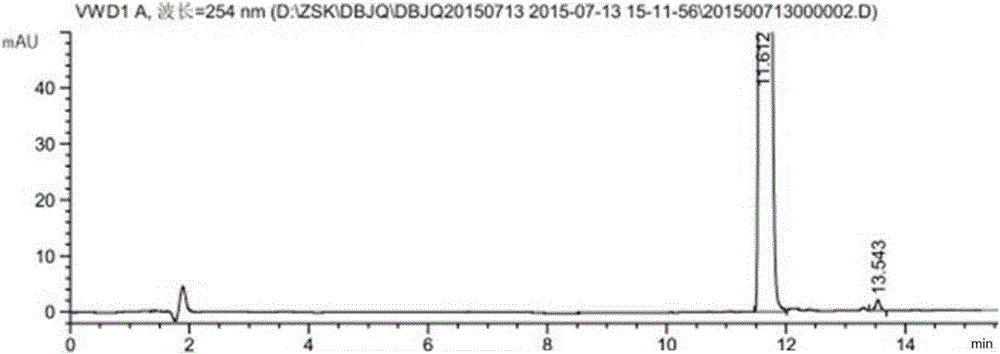
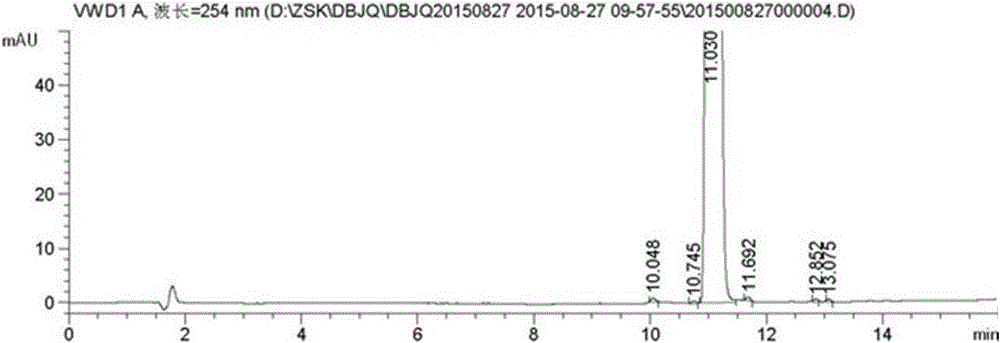
Image
Examples
Embodiment 1
[0034] Embodiment 1 prepares N-(4-cyanophenyl) aminoacetate ethyl ester (II)
[0035] At room temperature, add p-aminobenzonitrile (11.8g, 0.1mol) and potassium carbonate (10.0g, 0.1mol) into acetonitrile (100ml), add ethyl bromoacetate (16.7g, 0.1mol) dropwise under stirring, and heat up to reflux , after reacting for 16h, cooled to room temperature, filtered, concentrated to remove the solvent, the residue was stirred with water, filtered, washed twice with water, and recrystallized from toluene to obtain II (17.9g, 88%).
Embodiment 2
[0036] Example 2 Preparation of 3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl ) amino] ethyl propionate (III) method (one)
[0037] In a 500mL reaction flask, add ethyl 3-[N-(4-methylamino-3-aminobenzoyl)-N-(pyridin-2-yl)amino]propionate (I) (16.4g, 0.048mol ), ethyl N-(4-cyanophenyl)aminoacetate (II) (11.2g, 0.055mol) and 300mL DMF (N,N-dimethylformamide), stirred for 1h, heated to 120℃ and stirred for reaction 10h. After cooling down to room temperature, the reaction solution was poured into 1000ml of ice water to precipitate a solid, which was suction filtered, and the filter cake was washed 3 times with water to obtain the crude product III, which was recrystallized with ethyl acetate to obtain the pure product (18.3 g, 79%).
[0038] Preparation of 3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl)amino] The method of ethyl propionate (III) (two)
[0039] In a 500mL reaction flask, a...
Embodiment 3
[0044] Example 3 Preparation of 3-[[[2-[[(4-amidinophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl )amino] ethyl propionate (IV)
[0045] 3-[[[2-[[(4-cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl](pyridin-2-yl)amino]propane Ethyl acetate (III) (10.0 g, 0.021 mol) was added to 6 M ethanol solution of hydrogen chloride (50 ml), and stirred overnight at room temperature. The excess solvent was distilled off, the residue was dissolved in 40ml ethanol solution, ammonium carbonate (19.2g, 0.2mol) was added, and stirred overnight at room temperature. Filter to remove insoluble matter, and the filtrate is concentrated to dryness under reduced pressure. The residue was dissolved in 60 ml of a mixed solution of ethyl acetate and ethanol (5:1), and stirred at room temperature for 2 h. Suction filtration and drying gave compound IV hydrochloride (10.2 g, 92%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com