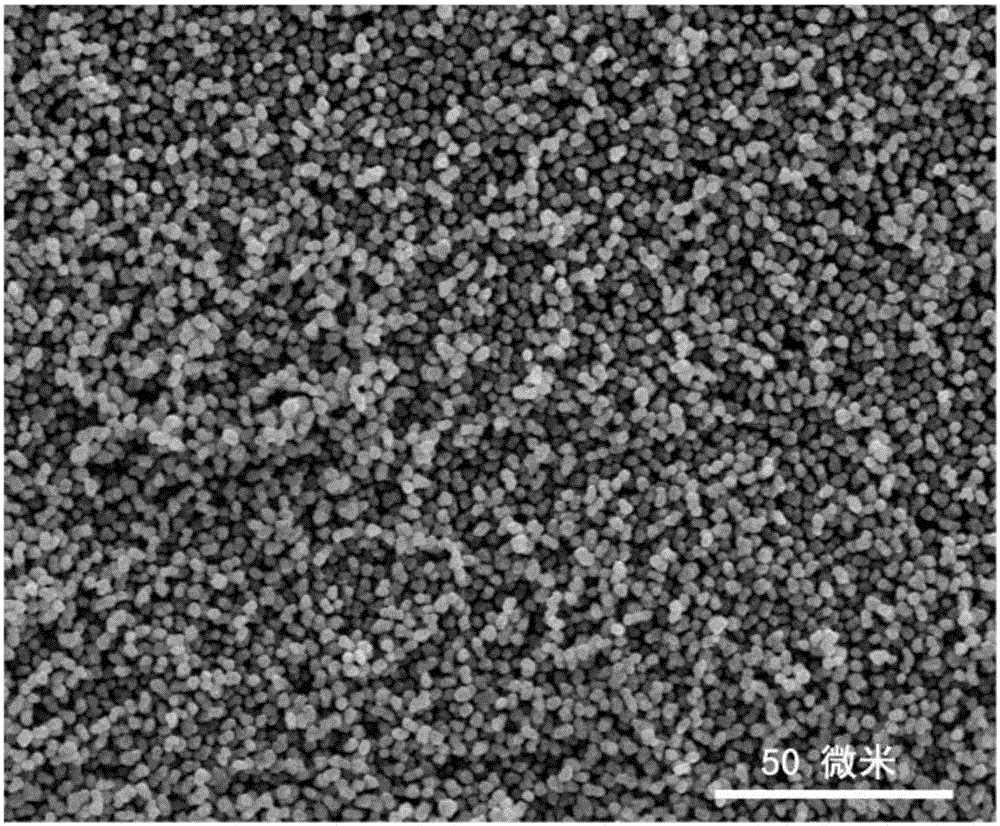
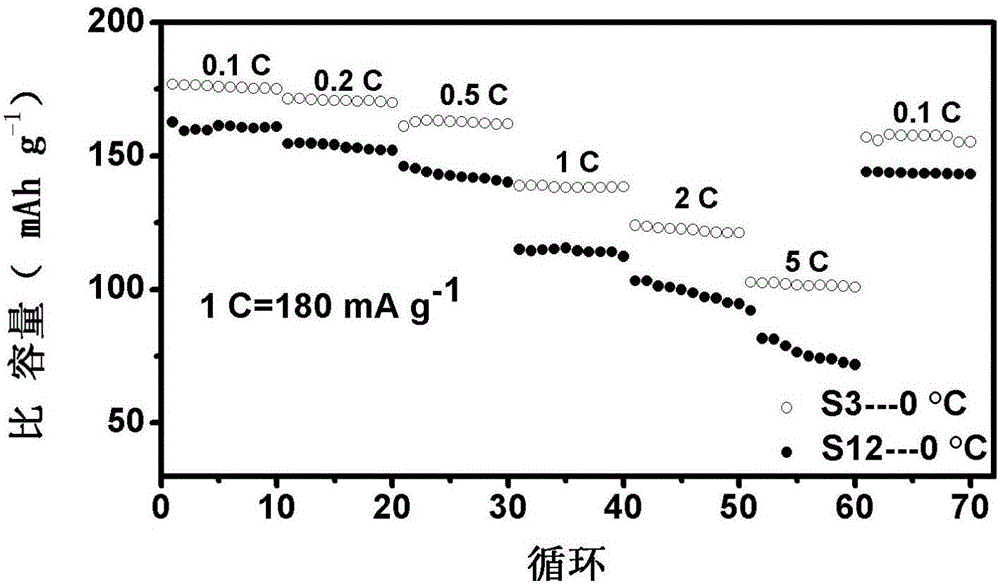
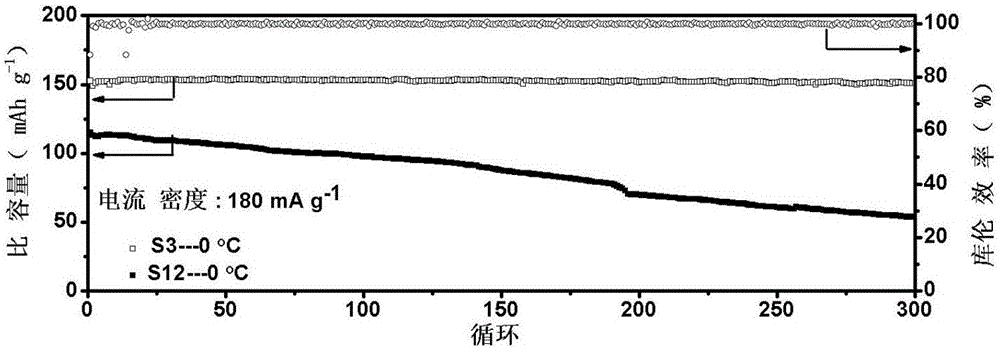
Low-temperature lithium ion battery anode material and method for preparing same
A technology for lithium-ion batteries and cathode materials, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor low-temperature performance of lithium-ion batteries and cannot meet the needs of the electric vehicle market, and is suitable for large-scale commercial production. The effect of low manufacturing cost and high specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also provides a method for preparing a low-temperature lithium-ion battery positive electrode material, comprising the following steps:
[0033] Step 1: Weigh nickel salt, cobalt salt, and manganese salt according to the stoichiometric ratio and dissolve them in deionized water. After the dissolution is complete, pass an inert gas to remove oxygen for 30 to 60 minutes to prepare a nickel, cobalt, and manganese salt solution;
[0034] Step 2: Prepare a mixed aqueous solution of complexing agent and precipitating agent, and pass inert gas to remove oxygen for 30-60 minutes;
[0035] Step 3: prepare the complexing agent aqueous solution as the bottom liquid and add it to the reaction kettle, pass the inert gas as the protective gas, and then add the nickel-cobalt-manganese salt solution obtained in step 1 into the reaction kettle dropwise under mechanical stirring, and drop Add the mixed aqueous solution of precipitating agent and complexing agent, an...
Embodiment 1
[0046] Weigh nickel sulfate, cobalt sulfate, and manganese sulfate respectively in a molar ratio of 5:2:3, and dissolve them in deionized water to prepare a total concentration of 2mol / L, and pass an inert gas to remove oxygen for 30 minutes; a nickel-cobalt-manganese salt solution is obtained;
[0047] Prepare 4mol / L sodium hydroxide as a precipitant, 2mol / L ammonia as a complexing agent, mix the two, the volume ratio of the precipitating agent and complexing agent is 2:1, pass through N 2 Deoxygenation for 40 minutes;
[0048] Prepare a 2mol / L ammonia solution as the bottom liquid and first add it to the reaction kettle, and pass N at the same time 2 As a protective gas, under mechanical stirring, the nickel-cobalt-manganese salt solution is added to the reaction kettle at a rate of 2L / h by means of a metering pump, and the mixed solution of the precipitant and complexing agent is added dropwise, and the reaction system is precisely controlled The pH value of the solution i...
Embodiment 2
[0054] Weigh nickel chloride, cobalt chloride, and manganese chloride respectively in a molar ratio of 6:2:2, and dissolve them in deionized water to prepare a total concentration of 1.5mol / L, and pass an inert gas to remove oxygen for 60 minutes; Cobalt manganese salt solution;
[0055] Prepare 4mol / L sodium hydroxide as a precipitant, 2mol / L ammonia as a complexing agent, mix the two, the volume ratio of the precipitating agent and complexing agent is 5:1, pass through N 2 Deoxygenation for 60 minutes;
[0056] Prepare a 2mol / L aqueous ammonia solution as the bottom liquid and first add it to the reaction kettle, and pass N 2 As a protective gas, under mechanical stirring, the nickel-cobalt-manganese salt solution is added to the reaction kettle at a dropping rate of 1L / h by means of a metering pump, and the mixed solution of the precipitating agent and complexing agent is added dropwise, and the reaction system is precisely controlled. The pH value of the solution was 11....
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com