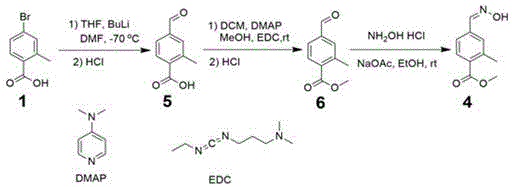
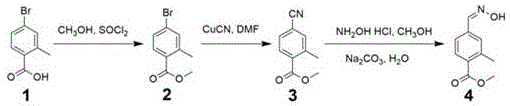
Method for preparing 2-methyl-4-formaldoxime methyl benzoate
A technology of methyl oximobenzoate and bromobenzoic acid, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of high production cost of raw materials, harsh reaction conditions, large safety risks, etc., and achieves low production cost, short synthesis route, and high technology. short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of methyl 2-methyl-4-bromobenzoate: Dissolve 1,5 g of 2-methyl-4-bromobenzoic acid in 30 ml of methanol, add 2 ml of thionyl chloride to it, and 70 o C was refluxed for 6 hours, the solution was cooled to room temperature, 30 ml of saturated sodium carbonate solution was added, fully stirred, 15 ml of ethyl acetate was used to extract twice, and the organic layer was successively washed with water 10 ml twice, and with saturated brine 10 ml. Dry over magnesium sulfate, filter, and concentrate under reduced pressure to obtain brown-red methyl 2-methyl-4-bromobenzoate 2, 5.17 g, yield 97%.
[0026]
[0027] (2) Preparation of methyl 2-methyl-4-cyanobenzoate: Add 20 ml DMF to dissolve 2-methyl-4-bromobenzoic acid methyl ester 2, 5.17 g obtained in step (1), and then Add 10.2 g of cuprous cyanide, stir, the solid matter dissolves quickly, the solution is clear, and heated to 160 o C was reacted for 12 hours, cooled to room temperature, the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com