Manganese peroxidase MNP-2, gene and applications thereof
A technology of manganese peroxidase and enzyme manganese peroxide, applied in the field of genetic engineering, can solve problems such as hindering large-scale application, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Cloning of Irpex lactus manganese peroxidase coding gene MNP-1
[0046] Extraction of total RNA from Irpex lactus:
[0047] Collect the mycelia cultured in liquid for 5 days, press dry on filter paper, and thoroughly grind into powder in liquid nitrogen; add 800 μl Trizol reagent to 100 mg mycelia, blow and mix, and place at room temperature for 5 minutes; add 200 μl chloroform, and shake vigorously for 15 seconds , placed at room temperature for 3 minutes, centrifuged at 4°C, 12,000 rpm for 15 minutes; aspirated the supernatant, added an equal volume of isopropanol, mixed well, placed at room temperature for 10 minutes, and centrifuged at 4°C, 12,000 rpm for 10 minutes; discarded the supernatant, and washed the precipitate with 70% ethanol for 1 The second time, the precipitate was dried in the air for 5 minutes; an appropriate amount of RNase-Free deionized water was added to dissolve the RNA, and the RNA concentration was measured on the NanoDrop micronucle...
Embodiment 2
[0052] The preparation of embodiment 2 recombinant manganese peroxidase
[0053] The expression vector pET-28a(+) was double digested (BamHI and XhoI), and the gene MNP-2 encoding manganese peroxidase was double digested (BamHI and XhoI) to cut out the mature manganese peroxidase The gene fragment of the gene was connected with the expression vector pET-28a(+), and the recombinant plasmid pET28a-MNP-2 containing the Irpex lacteus manganese peroxidase gene MNP-2 was obtained and transformed into Escherichia coli DE3 to obtain the recombinant Escherichia coli strain BL21(DE3) / MNP-2.
[0054] In the same way, a recombinant expression vector containing the complete cDNA sequence of the signal peptide gene sequence was constructed and transformed into Escherichia coli.
[0055] Take the DE3 strain containing the recombinant plasmid, inoculate it in 40mL LB culture medium, shake it at 250rpm at 37°C for 12h, transfer it to 300mLLB medium at a ratio of 2%, and culture it at 250rpm ...
Embodiment 3
[0056] The activity analysis of embodiment 3 recombinant manganese peroxidase
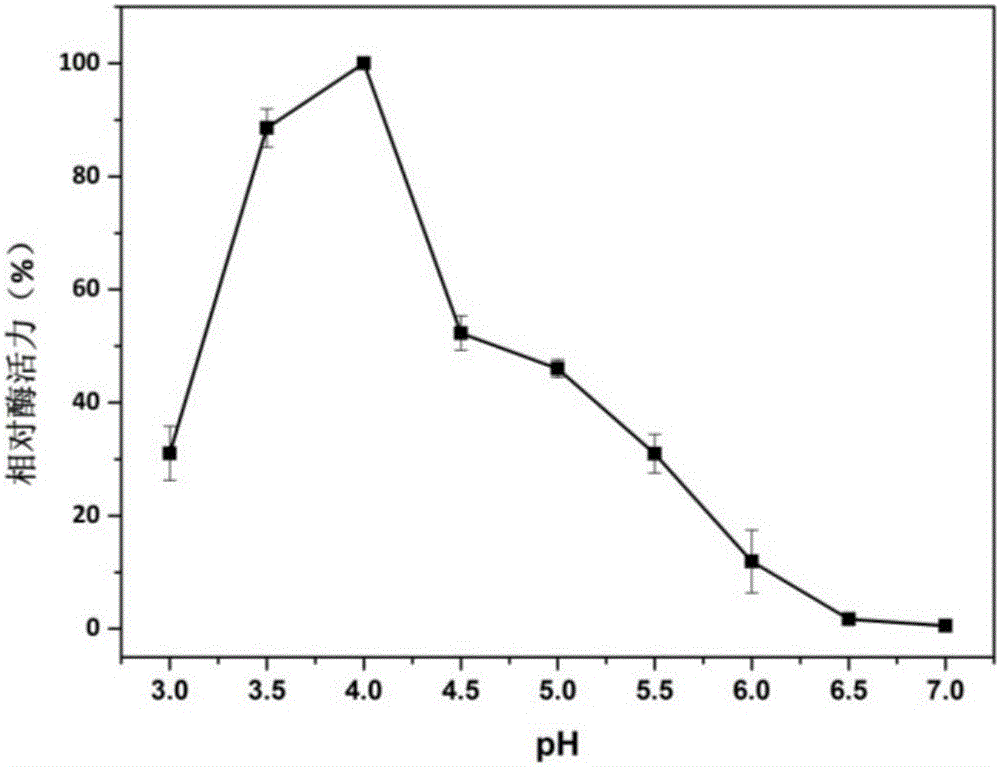
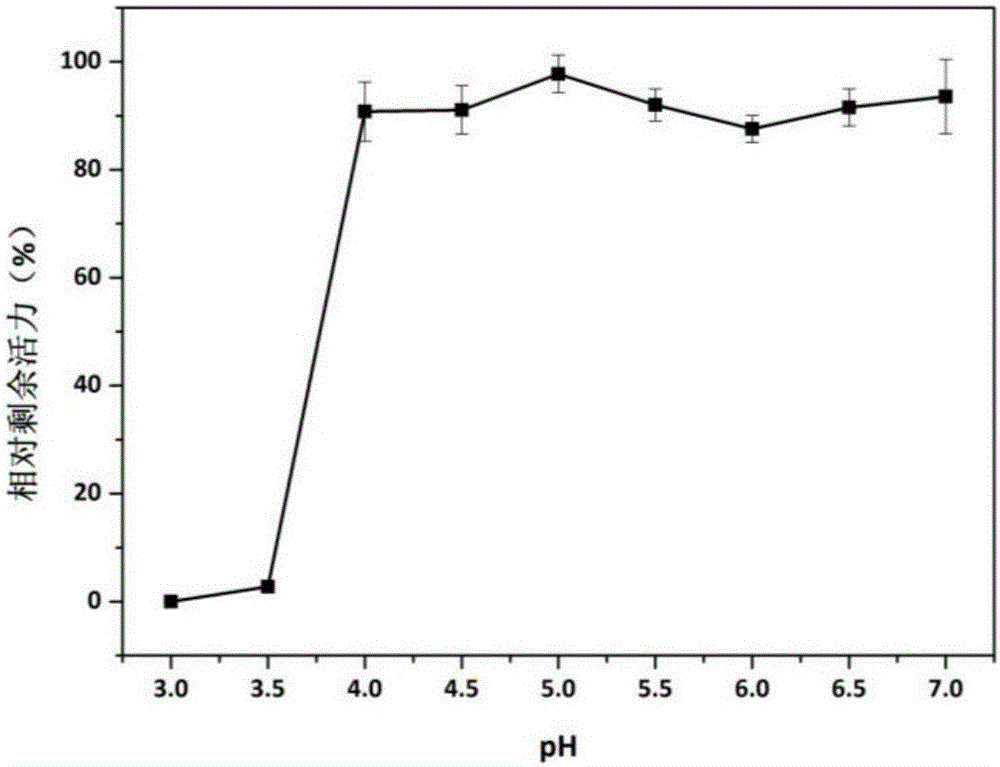
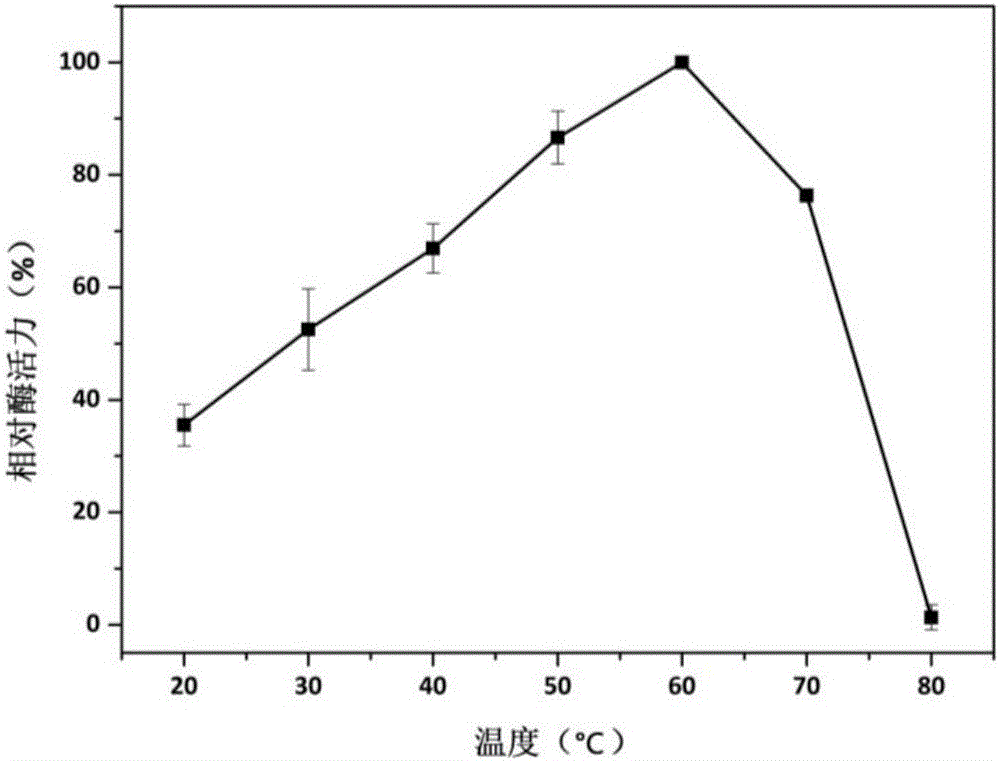
[0057] ABTS method (absorption coefficient 36000M -1 cm -1 ): The specific method is as follows: at pH 4.5 and 25°C, a 200μl reaction system includes 20μl of appropriately diluted enzyme solution, 20μl of 10mM ABTS, 50ul of 4mM manganese sulfate, and 50μl of 0.4mM hydrogen peroxide to start the reaction, and measure at 420nm Absorbance value, record a data every 20s, read 3min. One enzyme activity unit (U) is defined as the amount of enzyme required to oxidize 1 μmol ABTS per minute under given conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com