Method for synthesizing selenium-containing compound through decarboxylation coupled reaction one-pot method
A decarboxylation coupling and compound technology is applied in the field of synthesizing selenium compounds, which can solve the problems of limited sources of organic selenium and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
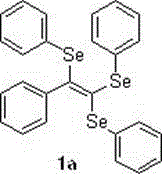
[0017] Synthesis of (2-phenylethylene-1,1,2-triyl) tris(phenyl selenide), its chemical reaction formula is:
[0018]
[0019] The synthesis method is as follows: add 0.5 mmol of phenylpropylic acid, 1.5 equivalents of diphenyl diselenide, 5% copper acetate, 1.2 equivalents of sodium carbonate and 10 mL of N-methyl pyrrolidone, at 90 o The reaction was carried out at C for 6 h, followed by TLC;
[0020] After the reaction is complete, cool to room temperature, pour the reactant into 15 mL of water, and extract 3 times with 20-30 mL of ethyl acetate, then wash once with saturated brine, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and purify by flash silica gel column chromatography, using petroleum ether as the eluent, and evaporate the eluent to dryness to obtain a yellow oily liquid 1a with a yield of 81%.
[0021] 1 H NMR (400 MHz, CDCl 3 ) δ 7.78 (dd, J = 7.3, 1.8 Hz, 1H), 7.64 – 7.58(m, 2H), 7.44 (dd, J = 6.6...
Embodiment 2
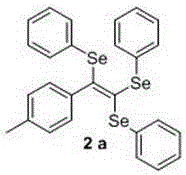
[0023] Synthesis of (2-(p-tolyl)ethylene-1,1,2-triyl)tri(phenylselenide), the chemical reaction formula is:
[0024]
[0025] The synthesis method is as follows: in a 25 mL round bottom flask, add 0.5 mmol p-tolylpropiolic acid, 1.5 equivalents of diphenyl diselenide, 5% copper chloride, 1.2 equivalents of sodium bicarbonate and 10 mL N-Methylpyrrolidone, at 90 o The reaction was carried out at C for 6 h, followed by TLC;
[0026] After the reaction is complete, cool to room temperature, pour the reactant into 15 mL of water, and extract 3 times with 20-30 mL of ethyl acetate, then wash once with saturated brine, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent under reduced pressure, and purify by flash silica gel column chromatography, using petroleum ether as the eluent, and evaporate the eluent to dryness to obtain yellow oily liquid 2a with a yield of 83%.
[0027] 1 H NMR (400 MHz, CDCl 3 ) δ 7.40 (dd, J = 7.3, 2.0 Hz, 2H), 7.28 – 7.21(...
Embodiment 3
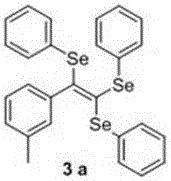
[0029] Synthesis of (2-(m-tolyl)ethylene-1,1,2-triyl)tri(phenylselenide), the chemical reaction formula is:
[0030]
[0031] The synthesis method is as follows: in a 25 mL round bottom flask, add 0.5 mmol m-tolylpropylic acid, 1.5 equivalents of diphenyl diselenide, 5% cuprous chloride, 1.2 equivalents of potassium carbonate and 10 mL N-Methylpyrrolidone, at 90 o The reaction was carried out at C for 6 h, followed by TLC;
[0032] After the reaction is complete, cool to room temperature, pour the reactant into 15 mL of water, and extract 3 times with 20-30 mL of ethyl acetate, then wash once with saturated brine, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent under reduced pressure, purify by flash silica gel column chromatography, use petroleum ether as the eluent, and evaporate the eluent to dryness to obtain yellow oily liquid 3a with a yield of 79%.
[0033] 1 H NMR (400 MHz, CDCl 3 ) δ 7.33 (s, 2H), 7.18 – 7.04 (m, 8H), 7.03 –6.95 (m, 3...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap