Method for preparing amoxicillin and phenylacetic acid from benzylpenicillin potassium
A technology for amoxicillin and penicillin potassium, which is applied in the field of preparation of amoxicillin and phenylacetic acid, can solve the problems of unfavorable amoxicillin synthesis yield, impact on amoxicillin product quality, low 6-APA concentration, etc., and achieve colored impurities Less, high yield, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
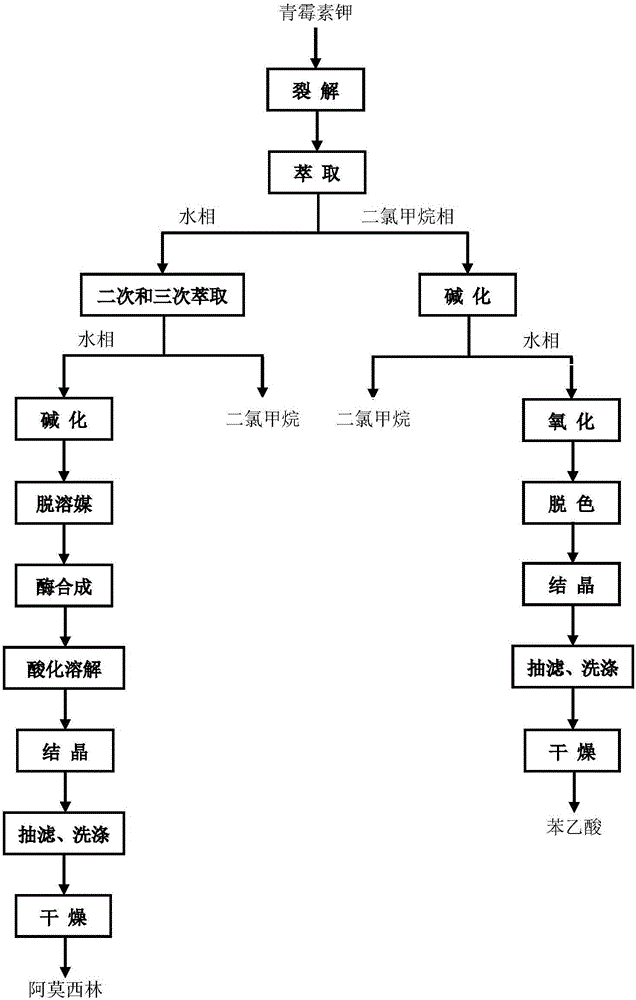
Image
Examples
Embodiment 1
[0047] Embodiment 1 The method for preparing amoxicillin and phenylacetic acid by penicillin potassium according to the present invention
[0048] (1) Penicillin Potassium Cleavage
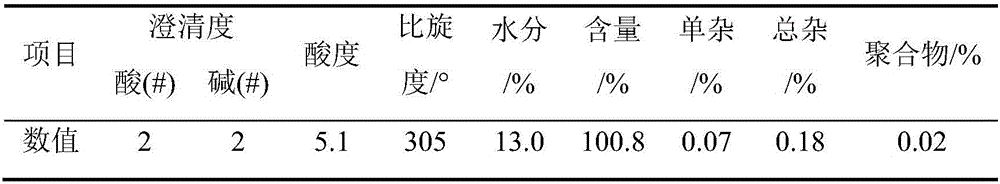
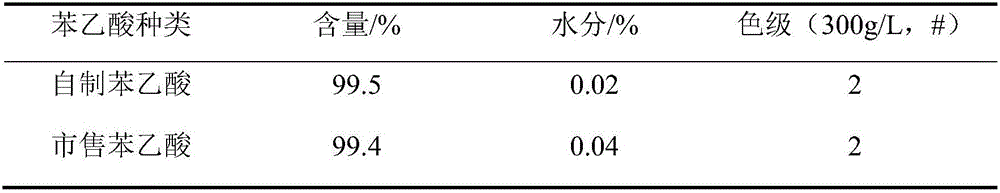
[0049] Weigh 9kg of penicillin potassium, add 90L of purified water, so that the concentration of penicillin potassium is 10%, then add 10.36kg of penicillin G acylase, the enzyme activity is 155u / g; start stirring, add 3mol / L ammonia water to the feed solution , the pH of the feed liquid was controlled to be 8.1, and the reaction temperature was controlled to be 29° C.; after reacting for 50 minutes, the reaction was stopped, and the feed liquid was separated from the penicillin G acylase to obtain 114 L of lysate, and the concentration of 6-APA was determined to be 45.60 g / L by sampling. The concentration of phenylacetic acid is 28.41g / L, and the cracking yield is 99.48%;
[0050] (2) Extraction
[0051] The lysate obtained in step (1) is cooled down. When the temperature drops to 5° C., add 5...
Embodiment 2
[0080] Embodiment 2 The method for preparing amoxicillin and phenylacetic acid by penicillin potassium according to the present invention
[0081] (1) Penicillin Potassium Cleavage
[0082] Weigh 9kg of penicillin potassium, add 75L of purified water, so that the concentration of penicillin potassium is 12%, then add 10.36kg of penicillin G acylase, the enzyme activity is 155u / g; start stirring, add 3.5mol / L to the feed solution Ammonia water, control the pH of the feed solution to be 8.0, and control the reaction temperature to be 35°C; react for 50 minutes, stop the reaction, separate the feed solution from penicillin G acylase to obtain 100 L of lysate, and take a sample to determine the concentration of 6-APA to be 51.66 g / L , the concentration of phenylacetic acid is 32.57g / L, and the cracking yield is 98.86%;
[0083] (2) Extraction
[0084] The lysate obtained in step (1) is cooled. When the temperature drops to 5° C., add 50 L of dichloromethane to the lysate. The te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com