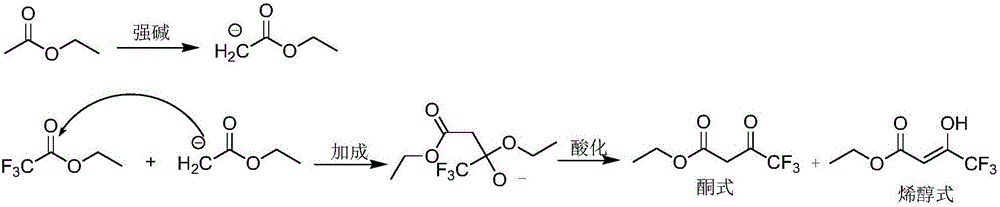
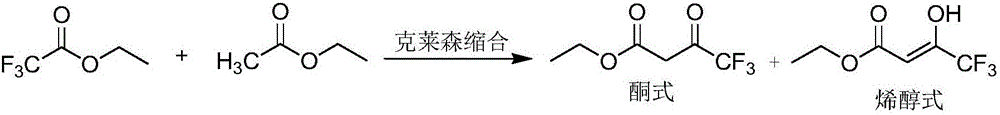
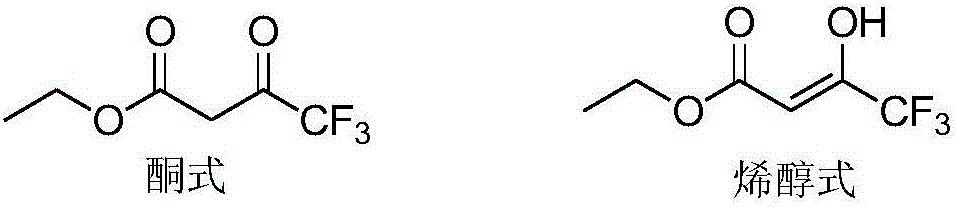Preparing method for ethyl 4,4,4-trifluoroacetoacetate
A technology of ethyl trifluoroacetoacetate and ethyl trifluoroacetate is applied in the field of preparation of ethyl 4,4,4-trifluoroacetoacetate, and can solve the problem that the end point of the dealcoholization process is difficult to control, the feeding process is difficult to control, and the solvent is difficult to control. Recycling energy consumption increases and other problems, to achieve the effect of novel process, convenient and easy-to-obtain raw materials, and shortened operation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] At 25°C, 200 mL of absolute ethanol was added to the reactor, followed by 510 g (1.5 mol) of 20% sodium ethoxide ethanol solution, and then 105.6 g (1.2 mol) of ethyl acetate. Cool the reaction liquid to 5-10°C, then start to dropwise add 142g (1.0mol) ethyl trifluoroacetate, and control the temperature at 10-20°C. After the addition, the temperature was raised to 60°C to react for 2 hours, and then the reaction solution was cooled to 10-15°C. Add 166.6 g (1.7 mol) of concentrated sulfuric acid dropwise to the reaction solution, control the temperature at 20-30° C., and react at 30° C. for 2.5 hours after dropping, to obtain a turbid solution containing sodium sulfate precipitate.
[0033] The sodium sulfate precipitate is removed by filtration, and the filter cake is washed with ethyl acetate, and the filtrate obtained is subjected to rectification under reduced pressure to obtain 157.0g ethyl trifluoroacetoacetate, and the yield is 85.3% (in terms of ethyl trifluoroac...
Embodiment 2
[0035] At 25°C, 100 mL of cyclohexane was added to the reactor, followed by 374 g (0.55 mol) of 10% sodium ethoxide ethanol solution, and then 57.2 g (0.65 mol) of ethyl acetate. Cool the reaction solution to 5-10°C, then start to drop 71g (0.5mol) ethyl trifluoroacetate, and control the temperature at 15-20°C. After the addition, the temperature was raised to 50°C to react for 3 hours, and then the reaction solution was cooled to 10-15°C. Add 59.2g (0.6mol) 37% concentrated hydrochloric acid dropwise to the reaction solution, control the temperature at 20-30°C, and react at 40°C for 1.5 hours after the dropwise completion to obtain a turbid solution containing sodium chloride precipitate.
[0036] The sodium chloride precipitate was removed by filtration, the filter cake was washed with ethyl acetate, and the obtained filtrate was subjected to rectification under reduced pressure to obtain 75.6 g of ethyl trifluoroacetoacetate, with a yield of 82.2% (in terms of ethyl trifluo...
Embodiment 3
[0038] At 25°C, 100 mL of tetrahydrofuran was added to the reactor, followed by 385.3 g (0.85 mol) of 15% sodium ethoxide ethanol solution, and then 70.4 g (0.8 mol) of ethyl acetate. Cool the reaction liquid to 5-10°C, then start to drop 99.4g (0.7mol) ethyl trifluoroacetate, and control the temperature at 10-20°C. After the addition, the temperature was raised to 40°C to react for 4 hours, and then the reaction solution was cooled to 10-15°C. Add 54 g (0.9 mol) of acetic acid dropwise to the reaction solution, control the temperature at 20-30° C., and react at 35° C. for 2.0 hours after the drop is completed, to obtain a turbid solution containing sodium acetate precipitate.
[0039]The sodium acetate precipitate is removed by filtration, and the filter cake is washed with ethyl acetate, and the filtrate obtained is subjected to rectification under reduced pressure to obtain 107.4g ethyl trifluoroacetoacetate, and the yield is 83.4% (in terms of ethyl trifluoroacetate), The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com