Method for separating camptothecin and 10-hydroxycamptothecin by adoption of rosin-based macromolecules
A hydroxycamptothecin and rosin-based technology, applied in the field of high performance liquid chromatography, can solve problems that have not been reported, and achieve good permeability, high column efficiency, and good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
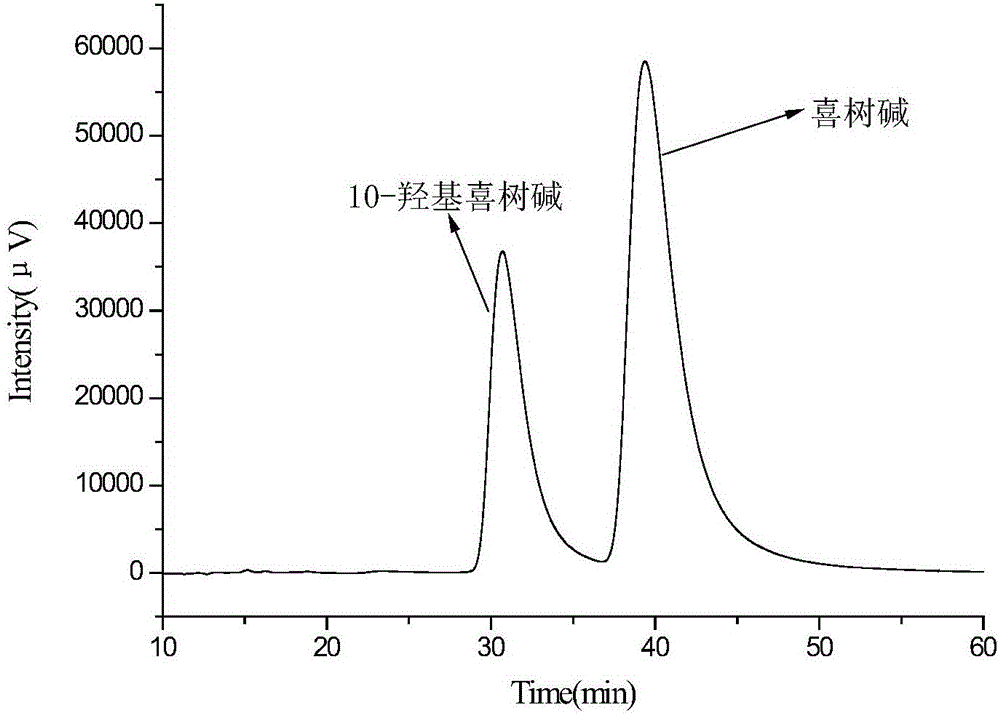
Embodiment 1
[0044] Add 200g of deionized water, 0.4g of sodium lauryl sulfate, and 4.0g of polyvinyl alcohol into a 250mL beaker (the mass ratio of ionized water, sodium lauryl sulfate, and polyvinyl alcohol is 100:0.2:2), Heat to 95°C to completely dissolve the polyvinyl alcohol and sodium lauryl sulfate to obtain an aqueous phase, which is transferred to a water bath to keep the temperature down to 55°C.
[0045] Weigh 3.00g of maleic rosin ethylene glycol acrylate, dissolve it in 50.0g of ethyl acetate, and use ultrasonic waves to promote dissolution. After the crosslinking agent is completely dissolved, add 0.50g of α-methacrylic acid and 1.00g of isooctane in sequence , 0.10g of azobisisobutyronitrile (the mass ratio of functional monomer, crosslinking agent, solvent, porogen, and initiator is 0.5:3:50:1:0.1), ultrasonically oscillated for 10min, dispersed evenly, and obtained oil Mutually.
[0046] Add the prepared oil phase to the water phase (the mass ratio of the oil phase and t...
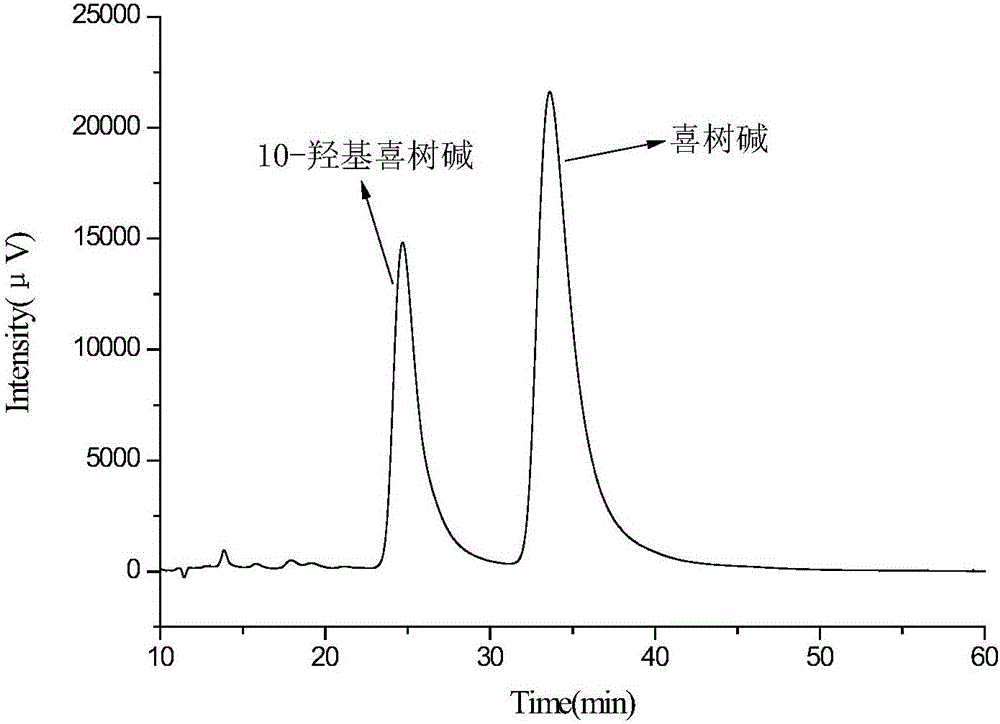
Embodiment 2
[0050] Add 400g deionized water, 2.00g sodium lauryl sulfate, and 12.00g polyvinyl alcohol into a 500mL beaker (the mass ratio of ionized water, sodium lauryl sulfate, and polyvinyl alcohol is 100:0.5:3), Heat to 95°C to completely dissolve the polyvinyl alcohol and sodium lauryl sulfate to obtain an aqueous phase, which is transferred to a water bath to keep the temperature down to 55°C.
[0051] Weigh 12.00g of maleic rosin ethylene glycol acrylate, dissolve it in 100.00g of ethyl acetate, and use ultrasonic waves to promote dissolution. After the cross-linking agent is completely dissolved, add 3.00g of methyl methacrylate and 5.00g of isooctyl Alkane, 2.00g of azobisisobutyronitrile (the mass ratio of functional monomer, cross-linking agent, solvent, porogen, and initiator is 3:12:100:5:2), ultrasonically oscillated for 10 minutes, dispersed evenly, and prepared oily phase.
[0052] Add the prepared oil phase to the water phase (the mass ratio of the oil phase to the wate...
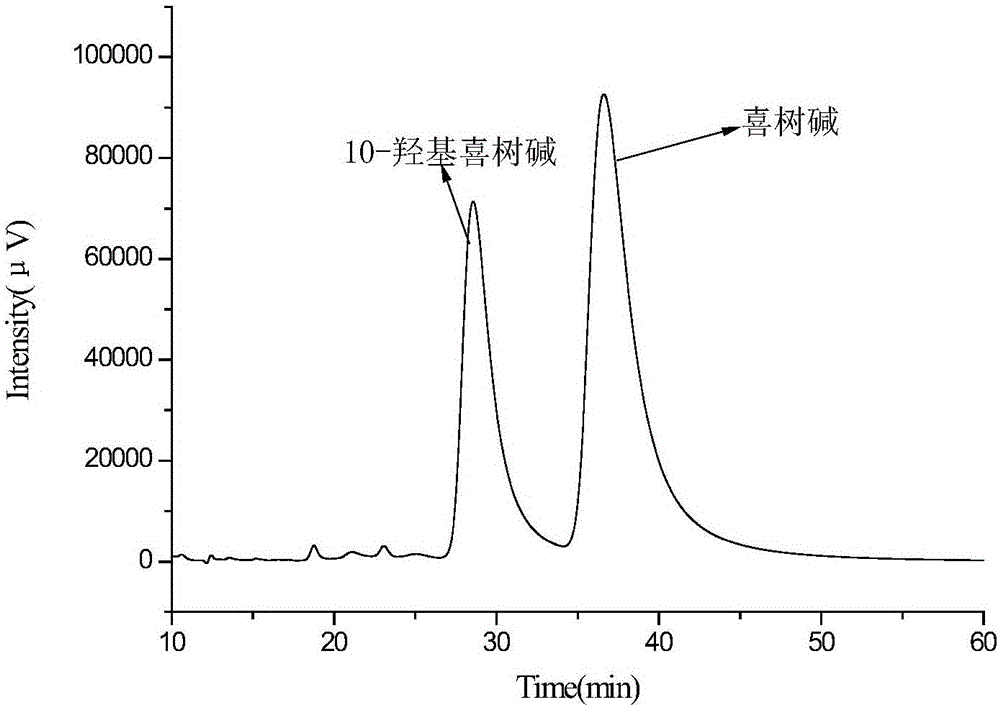
Embodiment 3
[0056] Add 300g of deionized water, 3.0g of polyvinyl alcohol, and 0.30g of sodium lauryl sulfate into a 400mL beaker (the mass ratio of ionized water, sodium lauryl sulfate, and polyvinyl alcohol is 100:0.1:1), Heat to 95°C to completely dissolve the polyvinyl alcohol and sodium lauryl sulfate to obtain an aqueous phase, which is transferred to a water bath to keep the temperature down to 55°C.
[0057] Weigh 3.25g of maleic rosin ethylene glycol acrylate, dissolve it in 80.0g of ethyl acetate, and use ultrasonic waves to promote dissolution. After the cross-linking agent is completely dissolved, add 1.55g of α-methacrylic acid and 3.0g of isooctane in sequence , 1.0g of azobisisobutyronitrile (the mass ratio of functional monomer, crosslinking agent, solvent, porogen, and initiator is 1.55:3.25:80:3:1), ultrasonically oscillated for 10min, dispersed evenly, and obtained oil Mutually.
[0058] Add the prepared oil phase to the water phase (the mass ratio of the oil phase to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com