Application of ursolic acid conjugate having anticancer activity and serving as drug carrier or molecular probe carrier
A technology of ursolic acid conjugates and molecular probes, applied in the field of biomedicine, can solve problems such as other diseases, inflammation of liver and other organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
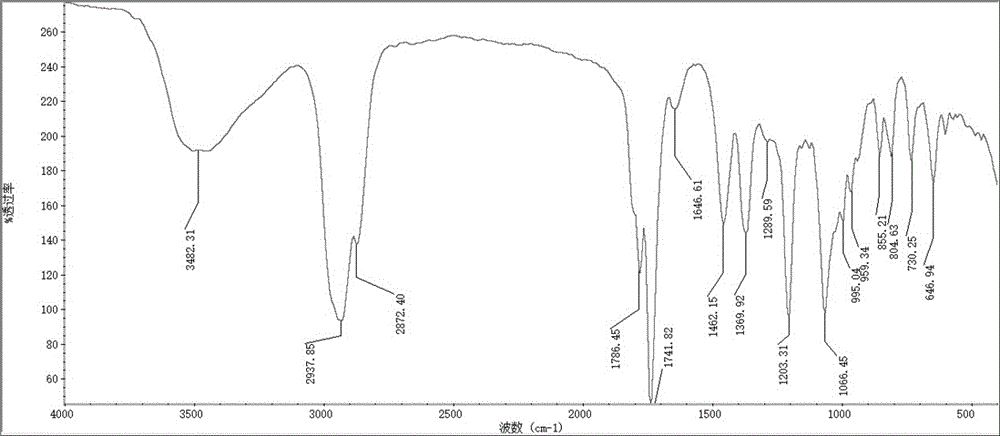
[0081] Synthesis of UA-Met, shown in structural formula VI, chemical name: (1S, 2R, 4aS, 6aS, 6bR, 10S, 12aR)-N-(N-(N,N-dimethylcarbamimidoyl)carbamimidoyl)-10-hydroxy-1 ,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b -octadecahydropicene-4a(2H)-carboxamide.
[0082] At room temperature, 200 mg of UA and 181 mg of DCC were stirred and dissolved with 3 ml of THF; 101 mg of NHS previously dissolved in 1.7 ml of acetonitrile was added dropwise to the reaction flask on an ice bath. The reaction was stirred at room temperature for 24 h. After the UA reaction was complete, the insoluble matter was removed by filtration, the solvent was evaporated under reduced pressure, and the activated UA was obtained by column chromatography. 100 mg of activated UA pure product and 30 mg of Met were stirred and dissolved in 5 ml of methanol. After reacting at room temperature for 24 h, the solvent was evaporated under reduced pressure, and the pure product of UA-Met...
Embodiment 2
[0089] Preparation method of UA-Met nanoparticles
[0090] Accurately weigh 0.00586 g of UA-Met powder, dissolve it in 1 ml of methanol, and ultrasonically dissolve it to form a 10 mM solution; take 20 uL of methanol solution and add it dropwise to an EP tube containing 180 uL (Note: drop Vortex during the addition process, 20 uL dropwise addition time is 20s), then. After sonication for 1 min, centrifugation at 12000 rpm for 5 min, the supernatant was UA-Met nanoparticles.
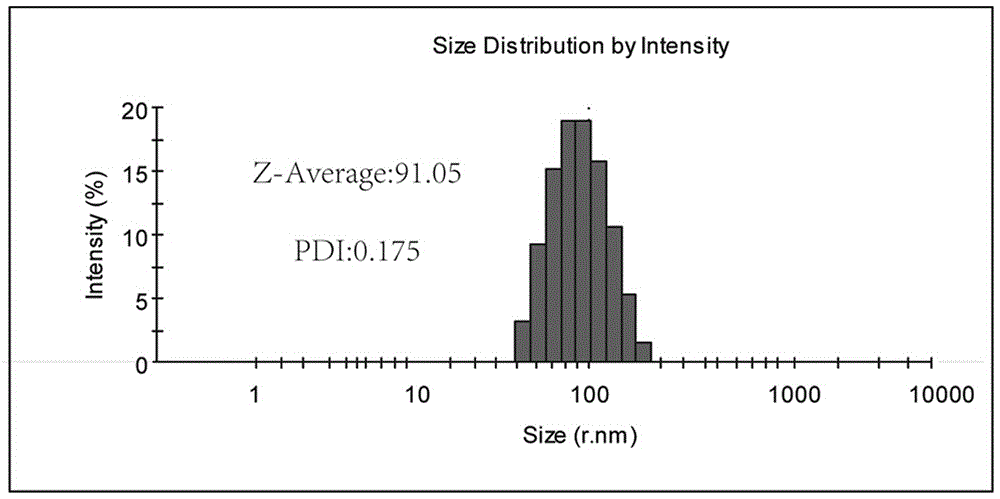
[0091] The average size of the particle diameter of the UA-Met nanoparticle aqueous solution prepared in this embodiment is about 110 nanometers, and the particle diameter diagram is as follows figure 2 shown.
Embodiment 3
[0093] Two parts of 200 ul of the nanoparticle aqueous solution prepared in Example 2 were placed in a cuvette, and then 800 ul of PBS solutions with a pH of 7.0 and a pH of 5.0 were added respectively, and then irradiated with a laser pointer to observe the phenomenon. The results are as follows: image 3 shown.
[0094] Such as image 3 As shown, the results show that the UA-Met nanoparticle aqueous solution maintains its nanostructure in the neutral pH=7.0 solution, and in the acidic condition pH=5.0, the nanoscale aqueous solution appears flocculent and the beam is dispersed. Numerous studies have shown that the tumor microenvironment is weakly acidic, which is necessary for the drug to be released in large quantities after entering the tumor microenvironment and responding to the pH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com