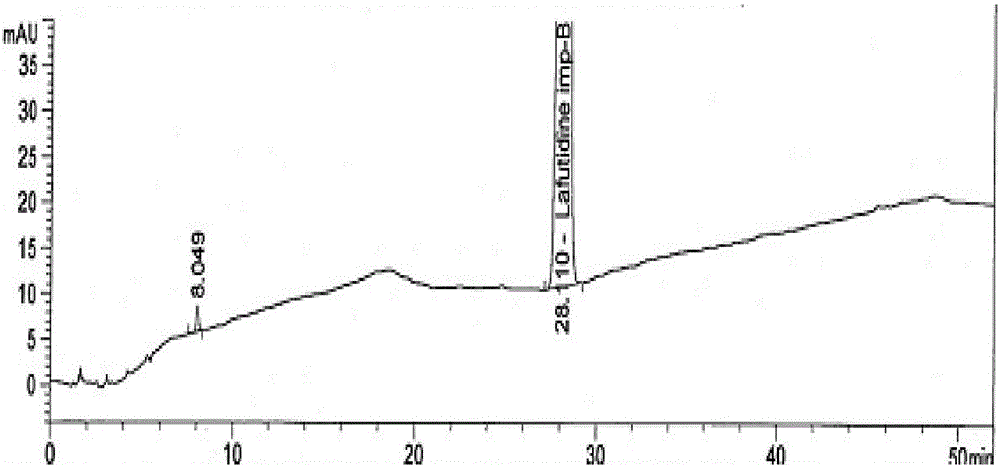
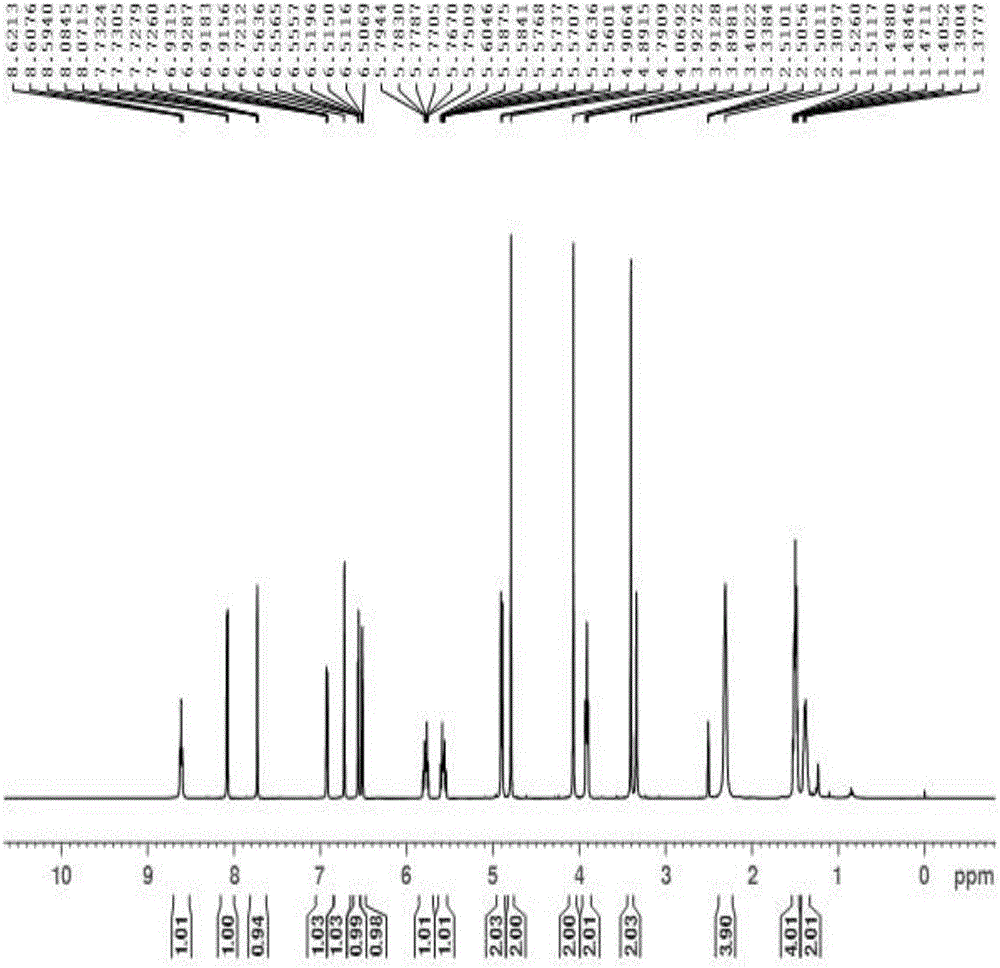
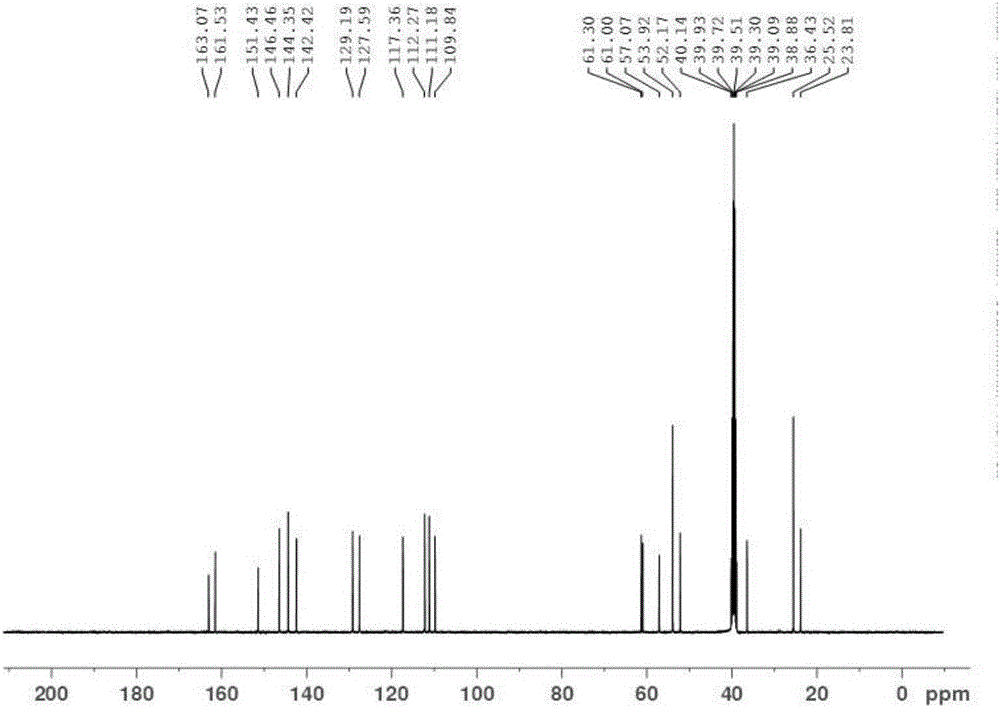
Synthetic method for Lafutidine oxide impurities
A technology for oxidizing impurities and synthesis methods, applied in the chemical field, can solve problems affecting product quality, etc., and achieve the effects of improving product purity and content, improving quality, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (2-(2-furyl methylsulfonyl) acetate-(4-nitrophenol) ester synthesis
[0031] Add 6.02g of 2-(2-furylmethylmercapto)acetic acid-(4-nitrophenol)ester (compound II, 0.02mol), 40mL of DMSO into a four-necked flask, stir at 20°C under temperature control, and add 6.16g of 2 - Iodosobenzoic acid (IBX, 0.22mol), temperature-controlled reaction for 12 hours. After the reaction was complete, 120 mL of water and 80 mL of dichloromethane were added, stirred for 0.5 minutes, and then separated. The organic layer was washed with water and saturated brine successively, the layers were separated, and the organic layer was spin-dried to obtain 5.2 g of an oil, which was recrystallized by adding acetone 26 mL, filtered, and dried to obtain (2-(2-furanmethylsulfonyl)acetic acid-( 4-nitrophenol) ester (compound III) 4.66g, yield 68.3%.
Embodiment 2
[0032] Example 2 2-[(2-furylmethyl)thionyl]-N-[4-[4-(1-piperidylmethyl)-2-pyridyl]oxygen-(Z)-2- Synthesis of butenyl]acetamide
[0033] 3.14g 4-[4-(N-piperidylmethyl)pyridine-2-oxygen]cis-2-butene-1-amine docking (compound IV, 0.012mol, 1eq), was added to 30mL ethyl acetate and 20mL5 %NaHCO 3 In the mixed solution, cool down to 0-5°C, add (2-(2-furanmethylsulfonyl)acetic acid-(4-nitrophenol) ester (compound III) 4.3g (0.0126mol, 1.05eq) and keep stirring 2h. After the reaction, the layers were separated, and the organic layer was washed with 2% NaHCO 3 , saturated saline, and water, and spin-dried the organic layer after washing to obtain 5.5 g of light green oil, add 50 mL of methyl tert-butyl ether, stir for 1 h at 30-40 ° C, cool to 0-5 ° C, stir for 1 h, and filter. Dry under reduced pressure to get 2-[(2-furylmethyl)sulfinyl]-N-[4-[4-(1-piperidylmethyl)-2-pyridyl]oxygen-(Z) -2-butenyl]acetamide (Compound I) crude product 4.35g, yield 81.3%.
Embodiment 3
[0034] Example 3 2-[(2-furylmethyl)thionyl]-N-[4-[4-(1-piperidylmethyl)-2-pyridyl]oxygen-(Z)-2- Purification of butenyl]acetamide
[0035] Add 4.2 g of the crude product to 30 mL of isopropanol, raise the temperature to 35-40 °C, and then gradually cool down to 5-10 °C after 0.5 h, keep stirring for 2 h, filter, and dry under reduced pressure to obtain the fine compound I 3.3 with a yield of 78.6%. Purity >99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com