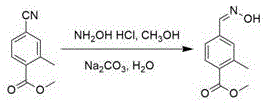4-formaldoxime benzoate derivative preparation method
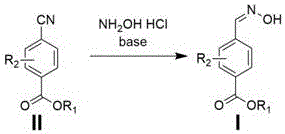
A technology of oximobenzoic acid and cyanobenzoic acid is applied in the directions of oxime preparation, organic chemistry and the like, can solve the problems of high production raw material cost, harsh reaction conditions, increase industrial cost and the like, and achieves low production cost, short synthesis route, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthetic route of one of the 4-formaldoximobenzoate derivatives (methyl 2-methyl-4-formaldoximobenzoate):
[0020]
[0021] Preparation of methyl 2-methyl-4-formaldoximobenzoate: Add 20 ml of methanol to dissolve methyl 2-methyl-4-cyanobenzoate (2.86 g), then add 1.15 g of hydroxylamine hydrochloride and 1.73 g anhydrous sodium carbonate, finally add 20 ml distilled water, at 70 o C was refluxed for 8 hours, cooled to room temperature, and concentrated under reduced pressure to obtain a crude product. The crude product was recrystallized with a mixed solvent of ethyl acetate and petroleum ether to obtain the pure target product, methyl 2-methyl-4-formaldoximobenzoate (2.53 g), with a yield of 87%. The appearance of the product is white crystal, the content is 99.2% (HPLC). [Mobile phase: water: methanol (5:2); detection wavelength: 240 nm; flow rate: 1ml / min; solution preparation: take 0.01g sample and dilute it to 25 ml with mobile phase; injection volume 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com