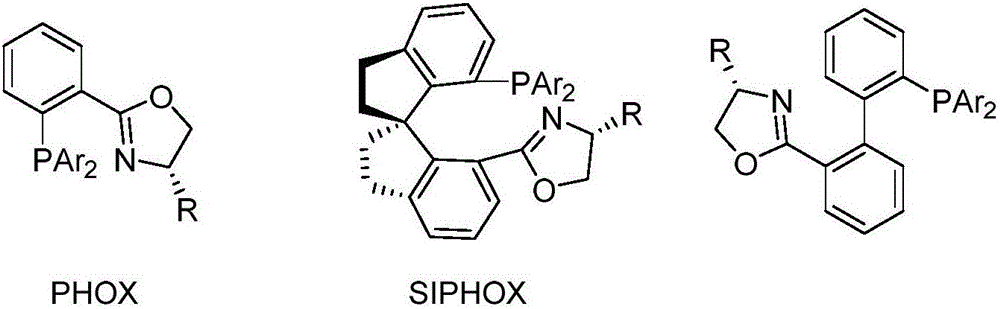
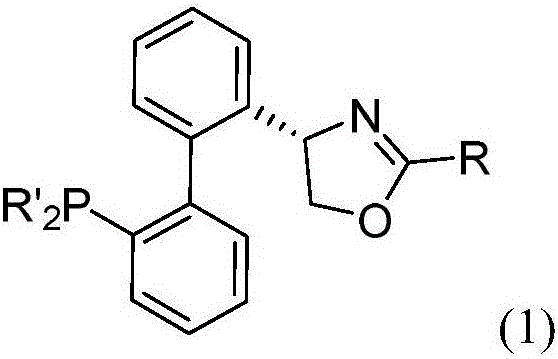
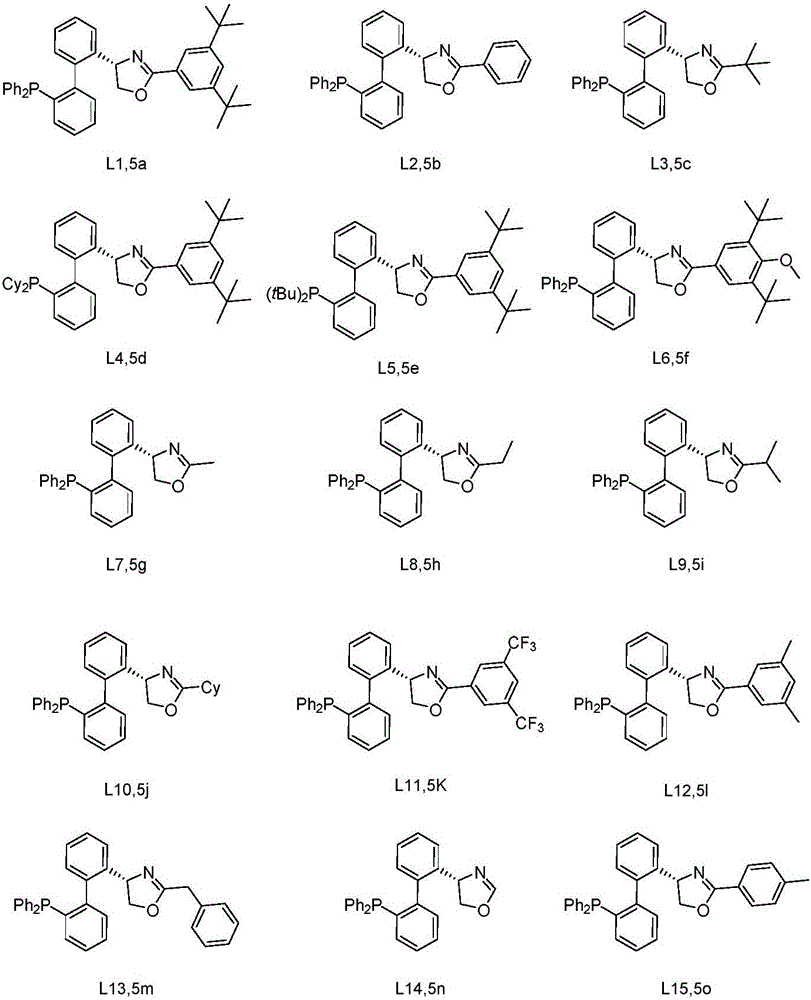
Chiral bidentate nitrogen-containing phosphine ligands and application thereof in asymmetric catalytic reaction
A technology of bidentate nitrogen and phosphine ligands is applied in the field of asymmetric catalysis synthesis, which can solve the problems of waste of ligands, high price, difficulty of optically pure axial chiral ligands, etc., and achieves avoidance of waste, short synthesis routes, and large industrial scale. The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of Ligand L1:
[0045]
[0046] L-phenylglycinol 1 (1.37g, 10mmol) was dissolved in 40mL of anhydrous THF, N 2 Protection, at -78°C, n-butyllithium (8mL, 2.5M n-pentane solution) was added dropwise, after the dropwise addition, stirred at -78°C for half an hour, then TBSCl (3.17g, 21.0mL) 20mL of THF solution was slowly added dropwise. After the dropwise addition was completed, it was slowly warmed to room temperature and then stirred overnight. After spinning THF away, N 2 Protection, under the condition of -78°C, add 50mL of anhydrous ether, add n-butyllithium (12mL, 2.5M n-pentane solution) dropwise, slowly rise to room temperature, stir at room temperature for 1 hour, then lower the temperature to - At 78°C, simple iodine (5.08g, 20.0mmol) was added, then raised to room temperature, and stirred at room temperature for 1 hour. After that with 10% Na 2 S 2 o 3 An aqueous solution (20 mL) was quenched, stirred for 10 minutes, extracted with ethyl ac...
Embodiment 2
[0065] Embodiment 2 Catalyst 6a catalyzes the asymmetric hydrogenation reaction of α-methylcinnamic acid:
[0066] In a glove box under an argon atmosphere, take catalyst 6a (3.6mg) and add it to a 5mL hydrogenation vial, then add the substrate α-methylcinnamic acid (32mg, 0.2mmol) and triethylamine (22mg, 0.2mmol) in sequence and 1 mL of dichloromethane. Put the hydrogenation vial into the hydrogenation reaction kettle, replace it with hydrogen three times and fill it with 30 atm H 2 , Reacted at room temperature for 4h. After the reaction was complete and hydrogen gas was released, the reaction mixture was passed through a short silica gel column. 1 The conversion rate (>99%) was determined by HNMR, and the enantioselectivity ee value (97%) of the reaction was determined by high performance liquid chromatography (HPLC).
Embodiment 3
[0067] Embodiment 3 Catalyst 6d catalyzes the asymmetric hydrogenation reaction of α-methylcinnamic acid:
[0068] In a glove box under an argon atmosphere, take catalyst 6d (3.6mg) and add it to a 5mL hydrogenation vial, then add the substrate α-methylcinnamic acid (32mg, 0.2mmol) and triethylamine (22mg, 0.2mmol) in sequence and 1 mL of dichloromethane. Put the hydrogenation vial into the hydrogenation reaction kettle, replace it with hydrogen three times and fill it with 30 atm H 2 , Reacted at room temperature for 4h. After the reaction was complete and hydrogen gas was released, the reaction mixture was passed through a short silica gel column. 1 The conversion rate (81%) was determined by HNMR, and the enantioselectivity ee value (81%) of the reaction was determined by high performance liquid chromatography (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com