Application of nitrogen-containing heterocyclic aromatic ester compounds in the preparation of drugs against Coxsackie virus type b3
A technology of ester compounds and Coxsackie virus, applied in antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of biological activity evaluation, etc., and achieve rapid economical and easy large-scale production The effect of simple promotion and synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] [Example 1] Synthesis of novel nitrogen-containing heterocyclic aromatic ester compounds
[0022] The present invention has synthesized the following 6 novel nitrogen-containing heterocyclic aromatic ester compounds, referring to the method of literature Tetrahedron Letters 2015, 56, 6136-6141, specifically using transition metal palladium as a catalyst, under the induction of the ortho position of pyridine, in the aromatic ring The ortho-position of aroyl is oxylated with hypervalent iodobenzene to obtain the final esterification product.
[0023]
Embodiment 2
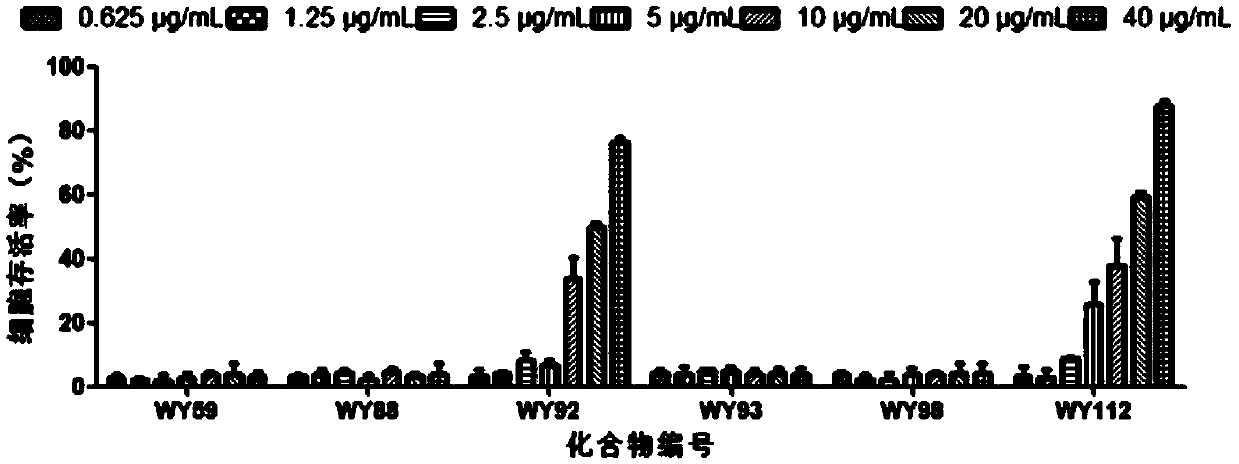
[0024] [Example 2] Evaluation of the anti-CVB3 activity of 6 compounds
[0025] 1. Test method:
[0026] 1.1 Toxicity of compounds to host Hep-2 cells
[0027] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the monolayer was grown in the incubator, the cell culture solution was discarded, and the cell maintenance solution containing different concentrations of the test compound was added to continue the culture. After 48 hours, the cytotoxicity was visually observed under the microscope and recorded respectively, and the cell survival rate was determined by the MTT method. SPSS 11.5 software calculates the median cytotoxic concentration (Median cyctoxic concentration, CC50) of the drug on the cells. Cell viability=(average OD of drug group 492 Value / average OD of cell control group 492 value) × 100%.
[0028] 1.2 Inhibitory activity of compounds against CVB3
[0029] Hep-2 cells were plated in 96-well plates at 37°C, 5% CO 2 After the monolayer was gro...
Embodiment 3
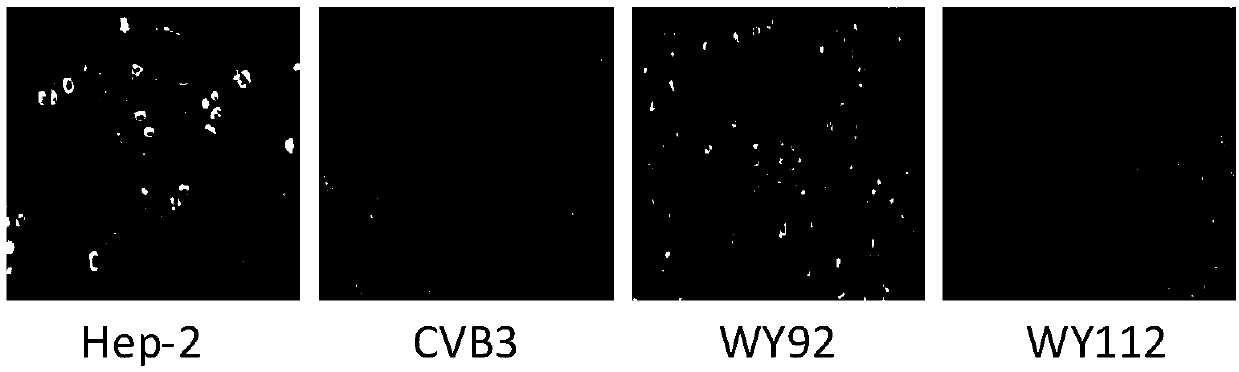
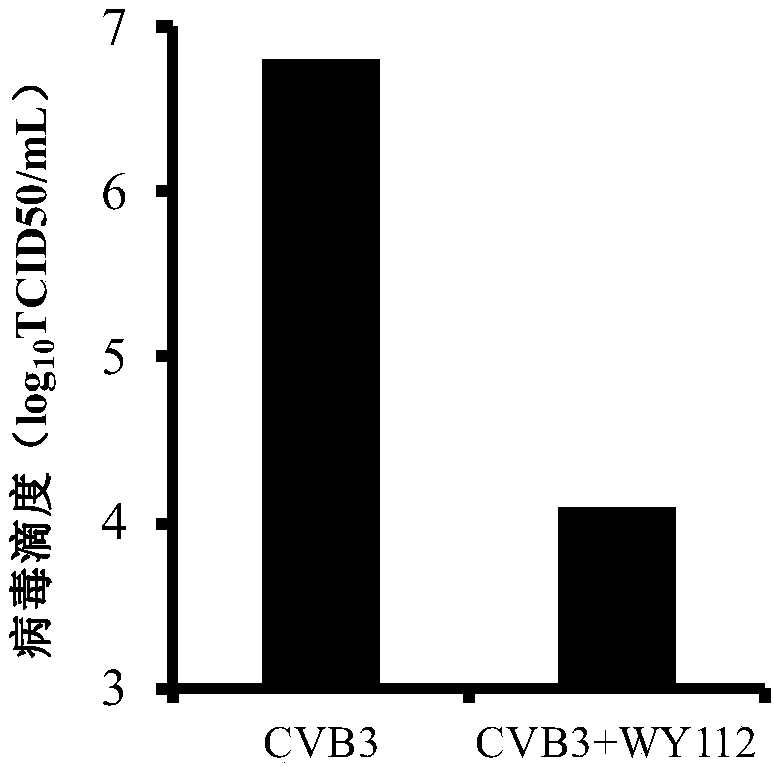
[0039] [Example 3] The inhibitory effect of WY112 on the production of CVB3 progeny virus
[0040] 1. Test method
[0041] Hep-2 cells in the logarithmic growth phase were plated in 24-well plates, and 100TCID after the monolayer was overgrown 50 CVB3 infected cells, incubated at 37°C for 1.5h, removed the virus solution, washed three times with PBS, and added cell maintenance solution containing 40μg / mL WY112. After 48 hours, the cells and supernatant culture fluid were collected, and after freezing and thawing three times at -20°C and 37°C, the titer of CVB3 virus was determined by the TCID50 method.
[0042] 2. Test results
[0043] Such as image 3 As shown, the virus titer of Hep-2 cells treated with 40 μg / mL WY112 was significantly lower than that of the virus control group, indicating that the compound had a strong inhibitory effect on the production of CVB3 progeny virus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com