Co(II)-based crystalline catalyst with layer pillaring structure and preparation method thereof
A catalyst and pillared technology, which is applied in the field of polymers, Co-based crystalline catalysts with a layered pillared structure and its preparation, can solve the problems of unpredictability in the synthesis process, uncontrollable assembly process, and poor stability of the catalyst. Good recycling effect, good selectivity and yield, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
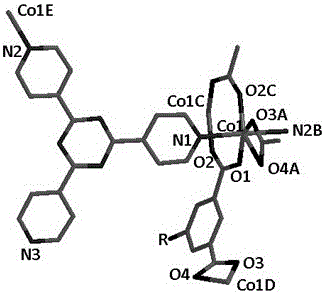
[0023] Embodiment 1: Synthesis and application of Co(II) crystalline catalyst 1
[0024] (1) According to 2,4,6-tris(4-pyridyl)-1,3,5-triazine and H 2 The molar ratio of BDC is 1:1, weighed and mixed to form a mixed compound;
[0025] (2) Weigh cobalt nitrate hydrate according to the ratio of 2:1 molar ratio between cobalt nitrate hydrate and 2,4,6-tris(4-pyridyl)-1,3,5-triazine in (1);
[0026] (3) The hydrated cobalt nitrate and the mixture were mixed in DMF / EtOH / H under ultrasound or stirring 2 O (DMF / EtOH / H 2 The volume ratio of O is 4:4:2) and mixed in a solvent to form a Co(II)-tpt precursor solution;
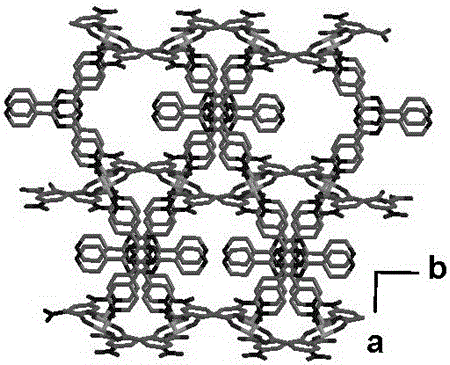
[0027] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle with a volume ratio of 1 / 3, and conduct a solvothermal reaction at 100°C for 72 hours. Finally, filter, wash, and dry to obtain a layered pillared Structure Co(II) crystalline material.
[0028]Use the Bruker APEX II diffractometer to collect diffraction intensity data to obtain a h...
Embodiment 2
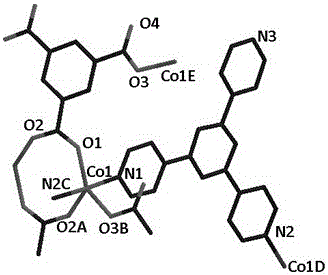
[0030] Embodiment 2: Synthesis and application of Co(II) crystalline catalyst 2
[0031] (1) According to 2,4,6-tris(4-pyridyl)-1,3,5-triazine with CH 3 -H 2 The BDC molar ratio is 1:1 and weighed, and it is a mixed compound after mixing;
[0032] (2) Weigh cobalt nitrate hydrate according to the ratio of 2:1 molar ratio between cobalt nitrate hydrate and 2,4,6-tris(4-pyridyl)-1,3,5-triazine in (1);
[0033] (3) Combine the hydrated cobalt nitrate and the mixture under ultrasonic or stirring in H 2 O / CH 3 CN (H 2 O and CH 3 The volume ratio of CN is 4:4) and mixed in a solvent to form a Co(II)-tpt precursor solution;
[0034] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle with a volume ratio of 1 / 3, and conduct a solvothermal reaction at 130°C for 72 hours. Finally, filter, wash, and dry to obtain a layered pillared Structure Co(II) crystalline material.
[0035] In a typical Knoevenagel condensation reaction, a flask containing a single...
Embodiment 3
[0036] Embodiment 3: Synthesis and application of Co(II) crystalline catalyst 3
[0037] (1) According to 2, 4, 6-tris(4-pyridyl)-1, 3, 5-triazine and NO 2 -BDC molar ratio is 1:1 and weighed, mixed into a mixed body;
[0038] (2) Weigh cobalt nitrate hydrate according to the ratio of 2:1 molar ratio between cobalt nitrate hydrate and 2,4,6-tris(4-pyridyl)-1,3,5-triazine in (1);
[0039] (3) Mix the hydrated cobalt nitrate and the compound in the solvent of isopropanol / water (the volume ratio of isopropanol and water is 4:2) under ultrasonic or stirring, and configure the precursor of Co(II)-tpt solution;
[0040] (4) Transfer the precursor solution obtained in (3) into a hydrothermal kettle with a volume ratio of 1 / 3, and conduct a solvothermal reaction at 130°C for 72 hours. Finally, filter, wash, and dry to obtain a layered pillared Structure Co(II) crystalline material.
[0041] The diffraction intensity data was collected on the Bruker APEX II diffractometer to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com