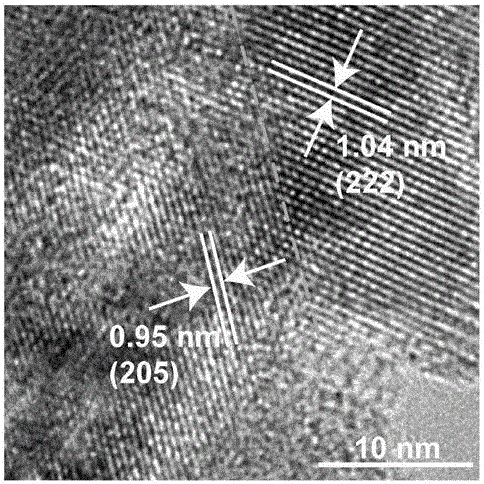
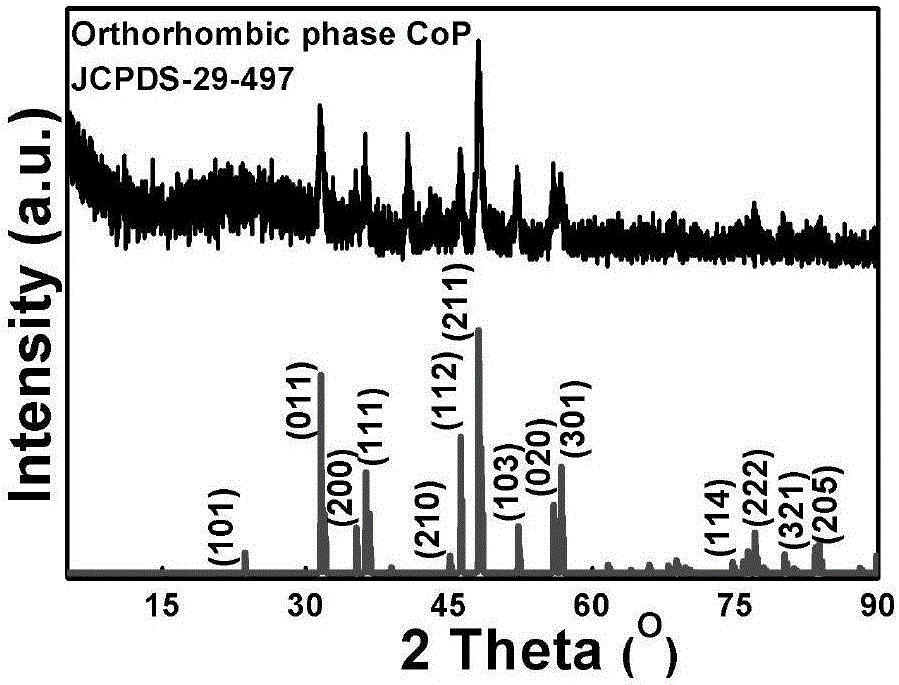
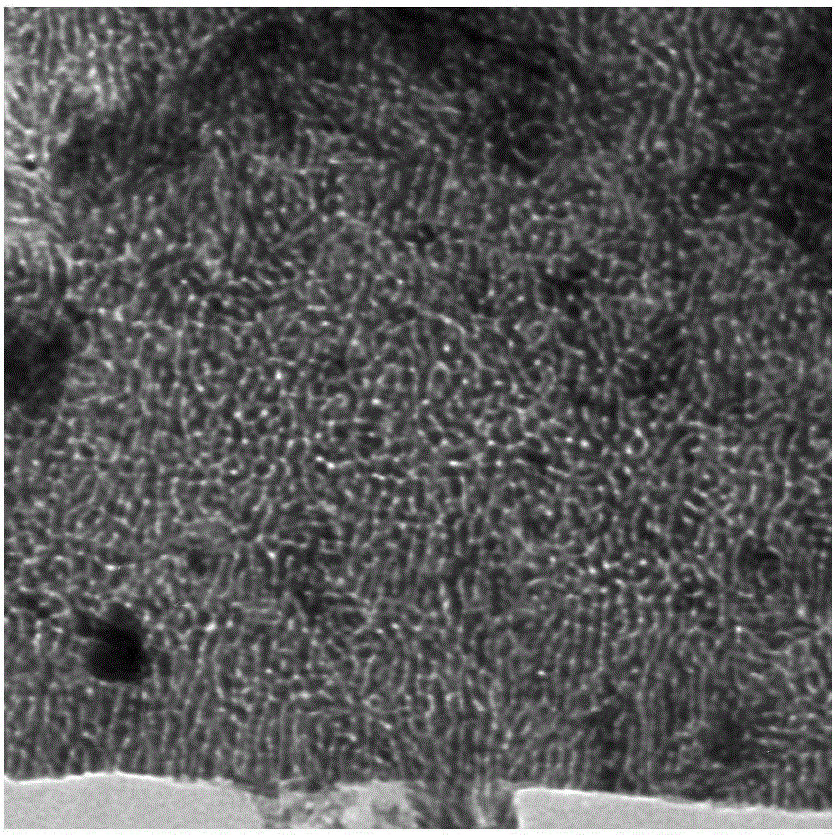
CoxPy porous nanometer sheet, and synthesis method and application thereof
A synthesis method and nanosheet technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of poor stability, high cost, low selectivity, etc., and achieve mild conditions, short reaction time, and reproducibility. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Co x P y (x=1; y=1) Synthesis of Porous Nanosheets and Their Electrocatalytic Hydrogen and Oxygen Evolution Properties
[0031] The specific synthesis process is as follows: at room temperature, in a clean and dry 250mL three-necked flask, add 5mol cobalt nitrate, 0.25-1.25g sodium dodecylsulfonate, 25mL deionized water and 25mL butanol, mix well, and then Add 3.0g of urea to the mixed solution to obtain the mixed solution A; then transfer the mixed solution A to a stainless steel reaction kettle with a polytetrafluoroethylene liner, react at 110°C for a period of time, and naturally cool to room temperature to obtain the product I, vacuum-dried at room temperature for 4 hours for analysis and characterization; the product I was then annealed, and in an oxygen-rich atmosphere, the temperature was raised to 250°C at a rate of 1-10°C / min, and then naturally cooled to At room temperature, product II is obtained (product II is an oxide of cobalt); then, in a mi...
Embodiment 2
[0038] Example 2: Co x P y (x=2; y=1) Synthesis of Porous Nanosheets and Their Electrocatalytic Hydrogen and Oxygen Evolution Performance
[0039] The specific synthesis process is as follows: at room temperature, in a clean and dry 250mL three-necked flask, add 5mol cobalt nitrate, 0.25-1.5g sodium dodecylsulfonate, 40mL deionized water and 40mL hexanol, mix well, and then Add 3.0g of urea to the mixed solution to obtain the mixed solution A; then transfer the mixed solution A to a stainless steel reaction kettle with a polytetrafluoroethylene liner, react at 110°C for a period of time, and naturally cool to room temperature to obtain the product I, vacuum-dried at room temperature for 4 hours for analysis and characterization; the product I was then annealed, and in an oxygen-enriched atmosphere, the temperature was raised to 350°C at a rate of 1-10°C / min, and then naturally cooled to At room temperature, product II was obtained; then, in a mixed solvent system (1-20 mL oc...
Embodiment 3
[0044] Example 3: Co x P y (x=1; y=3) Synthesis of Porous Nanosheets and Their Electrocatalytic Hydrogen and Oxygen Evolution Performance
[0045] The specific synthesis process is as follows: at room temperature, in a clean and dry 250mL three-necked flask, add 5mol cobalt acetate, 0.25-1.5g sodium stearate, 35mL deionized water and 40mL butanol, mix well, and then pour into the mixed solution Add 3.0g of urea to obtain mixed solution A; then transfer mixed solution A to a stainless steel reactor with a polytetrafluoroethylene liner, react at 110°C for a period of time, and then cool naturally to room temperature to obtain product I. Dry under vacuum for 4 hours for analysis and characterization; the product I is then annealed, and in an oxygen-enriched atmosphere, the temperature is raised to 450°C at a rate of 1-10°C / min, and then naturally cooled to room temperature to obtain the product II; then, in the mixed solvent system (1-20mL octadecene+1-10mL oleic acid), add 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com