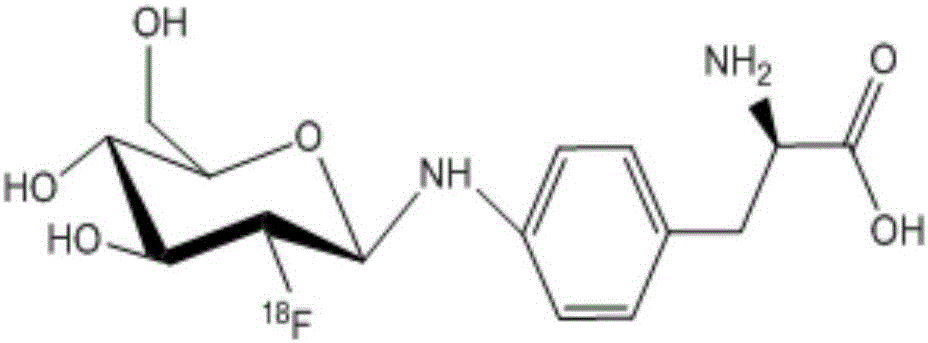
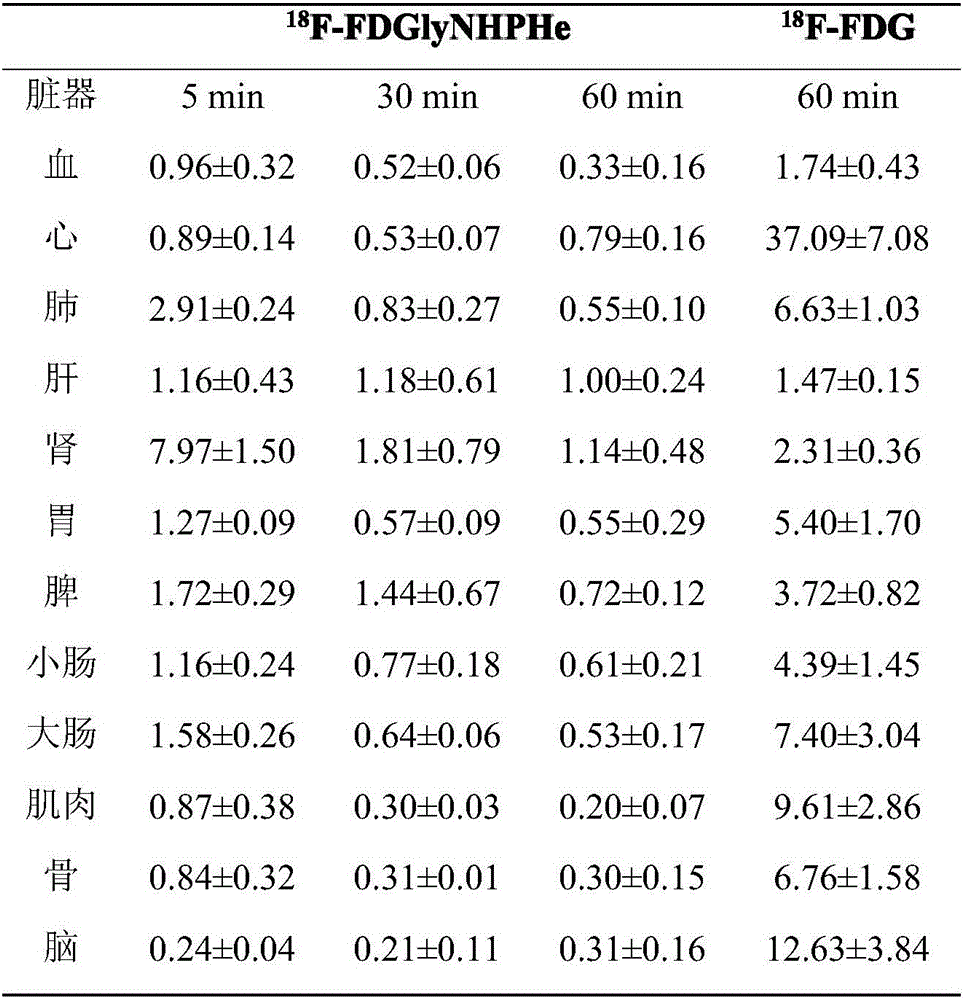
Fluorine-18 marked sugar-based amino acid compound, as well as preparation method and application thereof
A technology of amino acids and compounds, applied in the fields of radiopharmaceutical chemistry and clinical nuclear medicine, to achieve the effect of good biological properties, wide sources and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
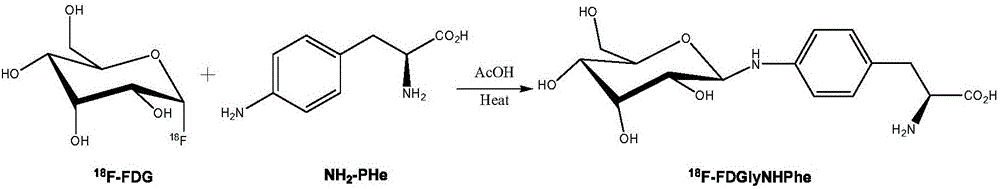
[0026] Embodiment 1: wet preparation [ 18 F] The preparation method of FDGlyNHPHe is as follows:
[0027] 0.5mL of 37MBq 18 F-FDG injection is placed in a closed container, 2 mL of acetonitrile is added twice, azeotropically dried under negative pressure, and then a mixture of 1.0 mg of p-aminophenylalanine in methanol and water (0.5 mL of methanol and 0.05 mL of water) and 50 μl of acetic acid, the container was sealed and reacted at 100° C. for 30 min; finally, the reaction solution was directly purified by preparative high-performance liquid chromatography. The conditions of preparative high performance liquid chromatography are: amino preparative column (250mm×10mm), mobile phase methanol:water=9:1, 3mL / min, UV=254nm. Collect the fractions between 10-17min, evaporate the solution to dryness, add PBS or normal saline, and then dilute and dissolve to obtain [ 18 F] The injection of FDGlyNHPHe is used after passing through a sterile filter membrane.
Embodiment 2
[0028] Embodiment 2: dry preparation [ 18 F] The preparation method of FDGlyNHPHe is as follows:
[0029] 0.5mL of 37MBq 18 Put F-FDG injection in a closed container, add 1.0mg of p-aminophenylalanine and 50μl of acetic acid at the same time, add 2mL of acetonitrile in two times, and azeotropically dry under negative pressure. After the solution is evaporated to dryness, the container is sealed. The reaction was carried out at 80° C. for 30 min; finally, the reaction solution was directly purified by preparative high performance liquid chromatography. The conditions of preparative high performance liquid chromatography are: amino preparative column (250mm×10mm), mobile phase methanol:water=9:1, 3mL / min, UV=254nm. Collect the fractions between 10-17min, evaporate the solution to dryness and add PBS to dilute and dissolve to obtain [ 18 F] The injection of FDGlyNHPHe is used after passing through a sterile filter membrane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com