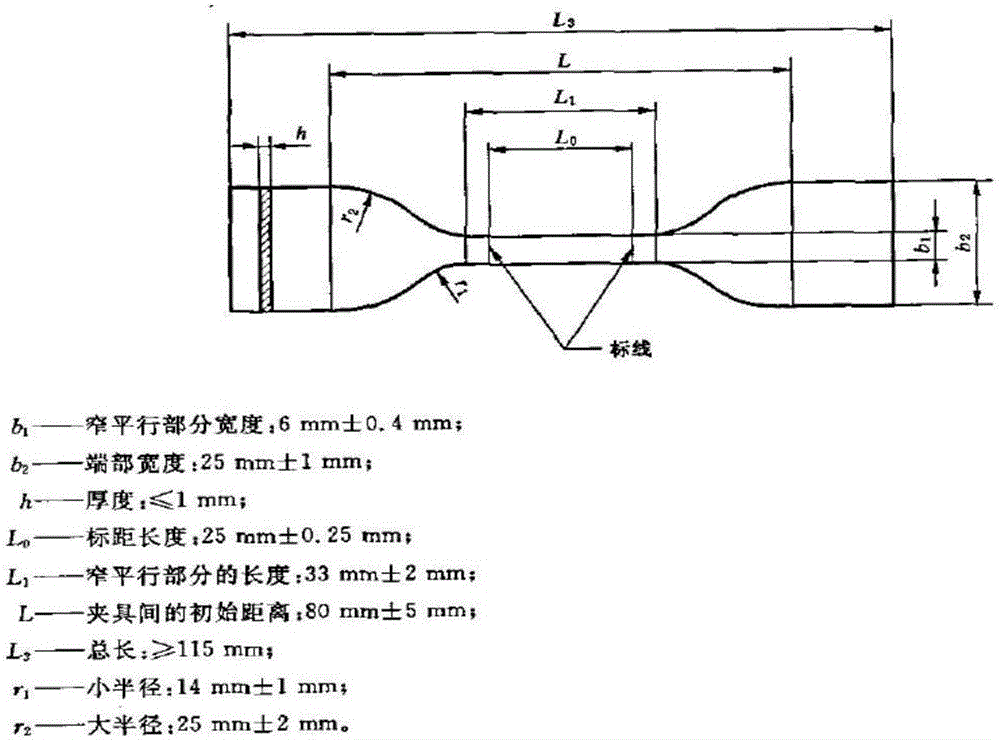
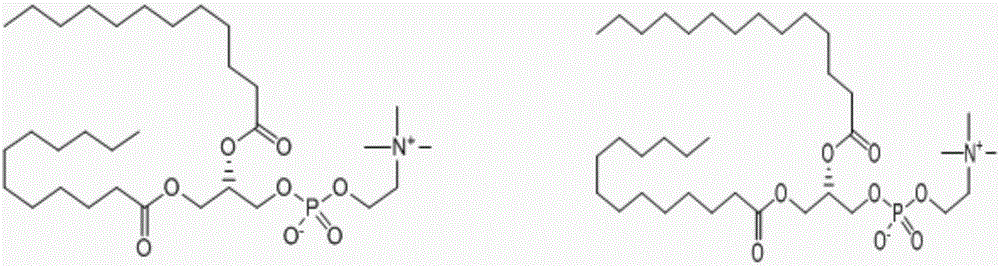
Polyester type polyurethane material with side chain containing phosphorylcholine group and preparation method thereof
A polyester polyurethane and polyurethane material technology, applied in the field of medical polymer materials, can solve the problems of limited biocompatibility, degradation of toxic substances, and difficulty in enrichment, etc., and achieve high biocompatibility, high mechanical Strength, thrombus avoidance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 0.01mol poly-L-lactide (PLLA, M n =2000) and 0.02mol HDI-BDO-HDI (dissolved in dimethyl sulfoxide: 10g / 30mL) were placed in a three-necked flask, protected by dry nitrogen, mechanically stirred, heated to 80 ° C, after 4.0 hours of reaction, cooled to 18 ℃, add 0.01mol Lys-PC, stir, when the viscosity of the system becomes too high to stir normally, add an appropriate amount of dichloromethane, after stirring for 1 hour, add dichloromethane to a concentration of about 15wt%, settle with glacial ether, suction filter, and store at room temperature Vacuum-dried to constant weight to obtain the polyurethane material A1 containing phosphorylcholine groups in the side chain.
[0041] Preparation of film material: Dissolve A1 in the organic solvent dioxane to make a solution with a concentration of 6.5% (g / mL), use a polytetrafluoroethylene mold to volatilize at 25°C under normal pressure for 80 hours, and remove the film from the mold After taking it off, dry it under norma...
Embodiment 2
[0043] 0.01mol polyε-caprolactone (PCL, M n =2000) and 0.02mol HDI-BDO-HDI (dissolved in dimethyl sulfoxide: 10g / 30mL) were placed in a three-necked flask, protected by dry nitrogen, mechanically stirred, heated to 85 ° C, after 3.5 hours of reaction, cooled to 18 ℃, add 0.01mol Lys-PC, stir, when the viscosity of the system becomes too high to stir normally, add an appropriate amount of dichloromethane, after stirring for 1 hour, add dichloromethane to a concentration of about 15wt%, settle with glacial ether, suction filter, and store at room temperature Vacuum-dried to constant weight to obtain the polyurethane material A2 containing phosphorylcholine groups in the side chain.
[0044] Preparation of film material: Dissolve A2 in the organic solvent chloroform to make a solution with a concentration of 5.5% (g / mL), use a polytetrafluoroethylene mold to volatilize at 24°C under normal pressure for 90 hours, and take the film from the mold After drying under normal temperatu...
Embodiment 3
[0046] 0.01mol polyε-caprolactone (PCL, M n =1500) and 0.02mol HDI-BDO-HDI (dissolved in dimethyl sulfoxide: 10g / 30mL) were placed in a three-necked flask, protected by dry nitrogen, mechanically stirred, heated to 85°C, and reacted for 3.5h, then cooled to 18°C ℃, add 0.01mol Lys-PC, and stir. When the viscosity of the system becomes too high to be stirred normally, add dichloromethane in an appropriate amount. After stirring for 1.5h, add dichloromethane to a concentration of about 15wt%, settle with glacial ether, and suction filter. Vacuum drying at room temperature to constant weight to obtain the polyurethane material A3 containing phosphorylcholine groups in the side chain.
[0047] Preparation of film material: Dissolve A3 in the organic solvent chloroform to make a solution with a concentration of 4% (g / mL), use a polytetrafluoroethylene mold to volatilize at 15°C under normal pressure for 60 hours, and take the film from the mold The membrane material is obtained by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com