Polyurea compositions and methods of use
A composition and prepolymer technology, applied in polyurea/polyurethane adhesives, chemical instruments and methods, adhesive types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
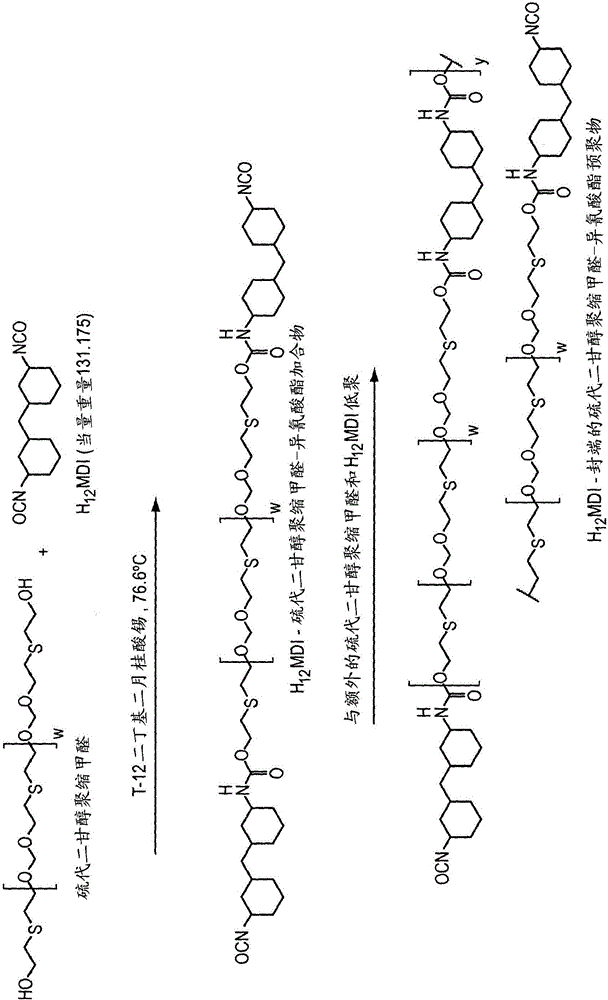
[0274] Polyoxymethylene polyol
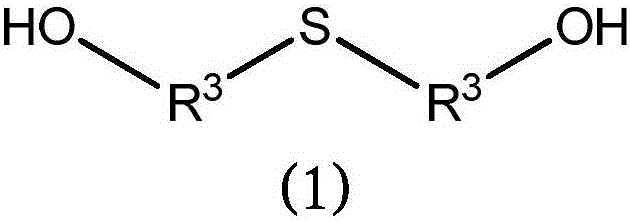
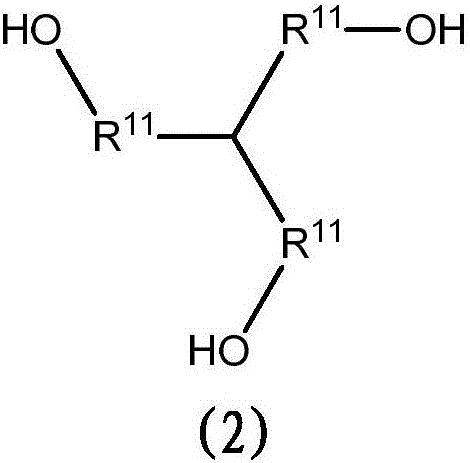
[0275] Thiodiethylene glycol (1833g), p-formaldehyde (95% purity) (360g), AMBERLYST TM 15 (319 g, obtained from Dow Chemical Company) and toluene (1000 mL) were charged to a 5 L 4 neck round bottom flask. The flask was equipped with a heating mantle, thermocouple, temperature controller and fitted with a reflux condenser, dropping funnel and a Dean-Stark adapter for nitrogen positive pressure inlet. The reaction was stirred under nitrogen, heated to 118 °C and held at 118 °C for approximately 7 h. During this period, the collected water was periodically removed from the Dean-Stark adapter. The reaction mixture was then cooled to room temperature and filtered through a coarse fused Buchner funnel (600 mL volume) with 9.0 cm diameter Whatman GF / A filter paper on the frit. The flask and filter cake were washed with 500 mL of toluene. Obtain filtrate. The filtrate was then vacuum dried using a 2L round bottom flask (rotary evaporator, 7 torr...
Embodiment 2
[0277] H 12 MDI-terminated polyoxymethylene-isocyanate prepolymer
[0278] The thiodiethylene glycol polyformal polyol (450 g) of Example 1 was charged into a 1000 mL 4-neck round bottom flask. The flask was equipped with a heating mantle, thermocouple, temperature controller, inlet to provide a positive pressure of nitrogen and a mechanical stirrer (PTFE paddle and bearing). The polyoxymethylene polyol was stirred and heated to 76.6°C (170°F) at about 200rpm, then added W(H 12 MDI) (99.5 g) and a solution of 0.01% dibutyltin dilaurate dissolved in methyl ethyl ketone (5.50 g). The reaction mixture was maintained at 76.6 °C for 7 h and then cooled to room temperature. A solution of 1% benzyl chloride dissolved in methyl ethyl ketone (5.50 g) was then added to the reaction mixture. The resulting thiodiethylene glycol polyformal-isocyanate prepolymer had an isocyanate content of 3.73% and a viscosity of 356 poise.
Embodiment 3
[0280] HDI-uretdione terminated polyoxymethylene-isocyanate prepolymer
[0281] The thiodiethylene glycol polyformal polyol (101 g) of Example 1 was charged into a 500 mL 4-neck round bottom flask. The flask was equipped with a heating mantle, thermocouple, temperature controller, inlet to provide a positive pressure of nitrogen and a mechanical stirrer (PTFE paddle and bearing). The polyoxymethylene polyol was stirred and heated to 76.6°C (170°F) at about 200rpm, then added XP-2730 (HDI-uretdione aliphatic polyisocyanate) (33.4 g) and a solution of 0.01% dibutyltin dilaurate dissolved in methyl ethyl ketone (1.4 g). The reaction mixture was maintained at 76.6 °C for 7 h and then cooled to room temperature. A solution of 1% benzyl chloride dissolved in methyl ethyl ketone (1.4 g) was then added to the reaction mixture. The resulting prepolymer had an isocyanate content of 3.41% and a viscosity of 695 poise.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| lap shear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com