Low-density amorphous carrier particles and its preparation method and application
A carrier particle, amorphous technology, which is applied in the directions of non-active medical preparations, pharmaceutical formulations, non-active components of polymer compounds, etc., can solve problems such as poor fluidity, achieve good fluidity, improve the effective deposition rate of drugs, The effect of high thermodynamic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Erythritol and hydroxypropyl-β-cyclodextrin were mixed according to different mass ratios (100:0, 80:20, 70:30, 60:40, 40:60, 30:70, 20:80, 0: 100) dissolved in water, respectively prepared into a solution with a concentration of 50mg / ml (the total concentration of erythritol and hydroxypropyl-β-cyclodextrin), and then using a spray dryer to spray dry the above solution to prepare carrier particles, The conditions of spray drying are as follows: the inlet air temperature is 130°C, the outlet air temperature is 85°C, the pump liquid rate is 7ml / min, the nozzle diameter is 0.71mm, the atomization pressure is 120Kpa, and the air flow rate is 0.60m 3 / h. The erythritol carrier particles obtained under these conditions were evaluated or characterized as follows.
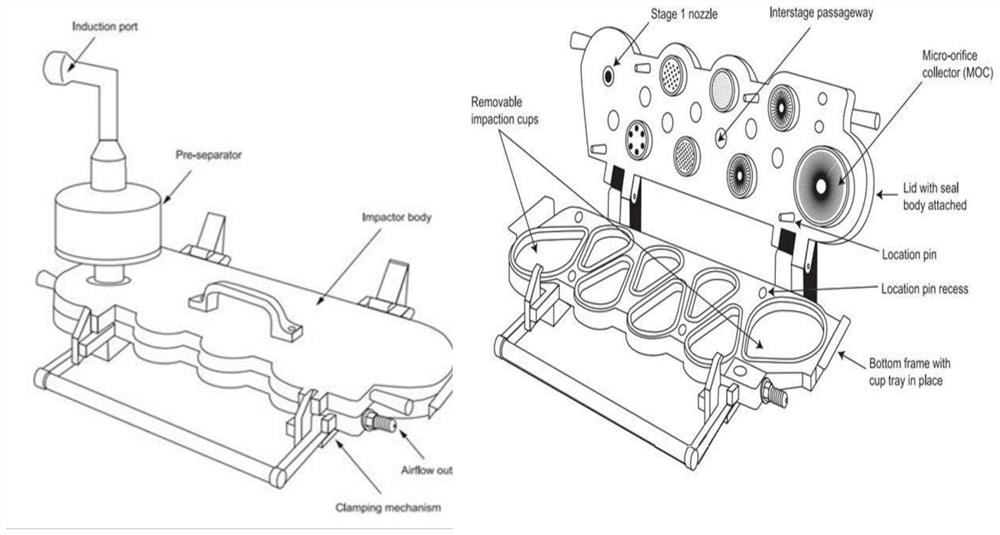
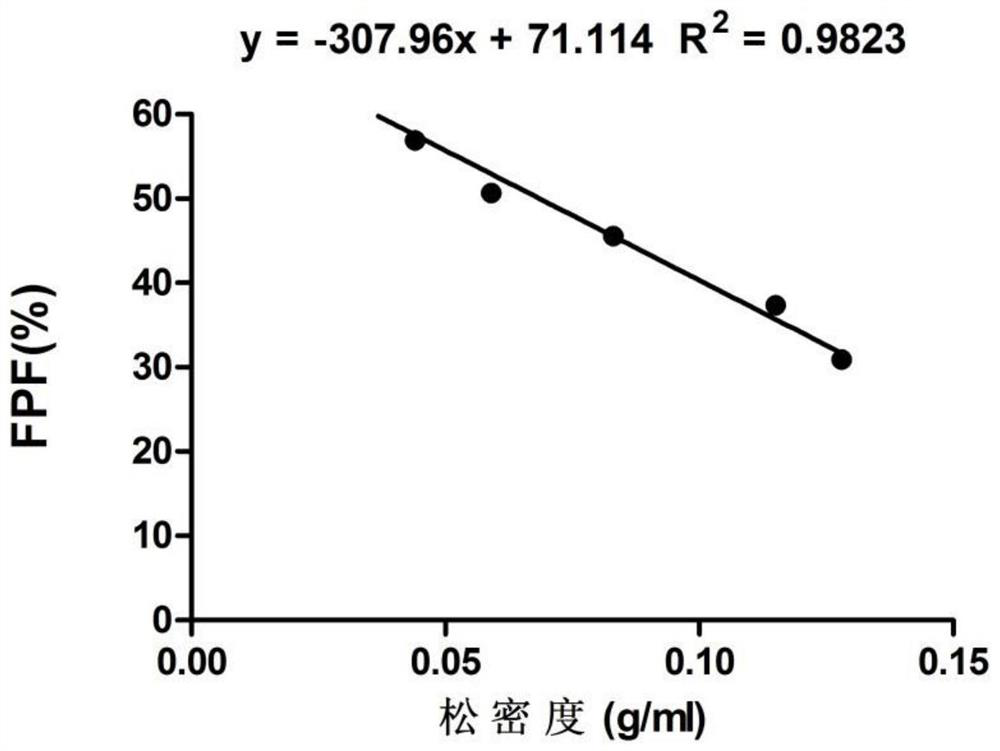
[0061] (1) Density measurement: bulk density and tap density are important properties of dry powder inhalation formulations. The specific method is: put a certain amount of powder (about 100mg) of known mass into...
Embodiment 2
[0084] Dissolve small molecule sugar alcohols (trehalose, raffinose, erythritol, xylitol, lactose) and hydroxypropyl-β-cyclodextrin in water at a mass ratio of 40:60, and prepare concentrations of 50mg / ml (the total concentration of small molecular sugar alcohol and hydroxypropyl-β-cyclodextrin), then adopt a spray dryer to spray dry the above solution to prepare carrier particles. The conditions of spray drying are as follows: the inlet air temperature is 130°C, outlet air temperature 85°C, pump liquid rate 7ml / min, nozzle diameter 0.71mm, atomization pressure 120Kpa, air flow 0.60m 3 / h.
[0085] (1) See Table 2 for the particle size, bulk density, crystal form, and glass transition temperature (Tg) of different composite carrier particles after spray drying (the test method is the same as in Example 1). The results showed that the particle size of the composite carrier particles prepared by spray drying with different small molecule sugar alcohols and hydroxypropyl-β-cyclo...
Embodiment 3
[0097] Small molecular sugar alcohol (raffinose or erythritol) and hydroxypropyl-β-cyclodextrin are dissolved in water at a mass ratio of 40:60, and the concentration is respectively prepared to be 50mg / ml (small molecular sugar alcohol and The total concentration of hydroxypropyl-β-cyclodextrin) solution, and then use a spray dryer to spray dry the above solution to prepare carrier particles. The conditions of spray drying are as follows: the inlet air temperature is 130°C, and the outlet air temperature is 85°C , the pump liquid rate is 7ml / min, the nozzle diameter is 0.71mm, the atomization pressure is 120Kpa, and the air flow rate is 0.60m 3 / h. Then the carrier particles were equally divided into 3 parts, and two of them were placed under accelerated stability conditions (40° C., 75% RH) for 5 days and 10 days respectively. Then the raffinose and erythritol carrier particles on day 0 and the raffinose and erythritol carrier particles after being placed under accelerated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com