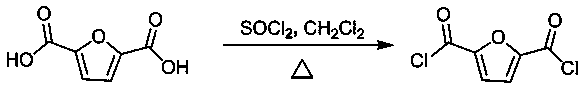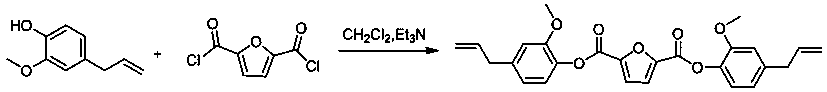A kind of modified bismaleimide resin and preparation method thereof
A bismaleimide resin, bismaleimide technology, applied in organic chemistry and other directions, can solve the problem that the BEG conversion rate is only 60%, the thermal performance and flexural modulus are reduced, and the crosslinking density of the cured product is reduced, etc. problems, to achieve the effect of easy industrial production, excellent thermal properties and rigidity, and high crosslinking density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of 2,5 furandicarboxylic acid chloride
[0032] See attached figure 1 , Which is the synthesis reaction formula for preparing 2,5 furandicarboxylic acid chloride in this example; the specific reaction conditions are as follows:
[0033] Mix 31.20g of 2,5 furandicarboxylic acid, 35.69g of thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05mL), stir and react at 80℃ for 3h, and cool naturally. At room temperature, the thionyl chloride was evaporated in a vacuum, and after drying, 2,5 furandicarboxylic acid chloride was obtained with a yield of 99.5%.
[0034] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
[0035] See attached figure 2 , It is the synthesis reaction formula for preparing all biomass-based bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate in this embodiment; the specific reaction conditions are as follows:
[0036] Dissolve 31.20g of eugenol and 24.29g of triethylamine as bases in 200mL of...
Embodiment 2
[0046] 1) Preparation of 2,5 furandicarboxylic acid chloride
[0047] Mix 31.20g of 2,5 furandicarboxylic acid, 35.69g of thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05mL), stir and react at 80℃ for 3h, and cool naturally. At room temperature, the thionyl chloride was evaporated in a vacuum, and after drying, 2,5 furandicarboxylic acid chloride was obtained with a yield of 99.5%.
[0048] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
[0049] Dissolve 32.84g of eugenol and 27.33g of triethylamine as bases in 300mL of dichloromethane and stir, add dropwise a solution of 2,5 furandicarboxylic acid chloride (19.30g) in dichloromethane (300mL) at -2.5±1℃ After the addition, the reaction solution was slowly raised to 20°C, and the reaction was continued for 3 hours; after the reaction, the dichloromethane was evaporated in a vacuum, washed with deionized water, and dried to obtain a total biomass-based bis(4-allyl- The yiel...
Embodiment 3
[0053] 1) Preparation of 2,5 furandicarboxylic acid chloride
[0054] 31.20g 2,5 furandicarboxylic acid, 35.69g thionyl chloride and N,N-dimethylformamide (DMF, catalyst, 0.05mL) were mixed, and the temperature was 70 o The reaction was stirred for 3 hours under the condition of C, cooled to room temperature naturally, and the thionyl chloride was evaporated in a vacuum. After drying, 2,5 furandicarboxylic acid chloride was obtained with a yield of 99.6%.
[0055] 2) Preparation of bis(4-allyl-2-methoxyphenyl)furan-2,5-dicarboxylate based on whole biomass
[0056] Dissolve 34.48g eugenol and 30.36g triethylamine as alkali in 500mL dichloromethane and stir, at -1±1 o Add a solution of 2,5 furandicarboxylic acid chloride (19.30g) in dichloromethane (500mL) under C. After the addition is complete, the reaction solution is slowly raised to 20°C, and the reaction is continued for 4 hours; after the reaction is complete, the dichloride is evaporated by vacuum Methane was washed with deioni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| flexural modulus | aaaaa | aaaaa |
| flexural modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com