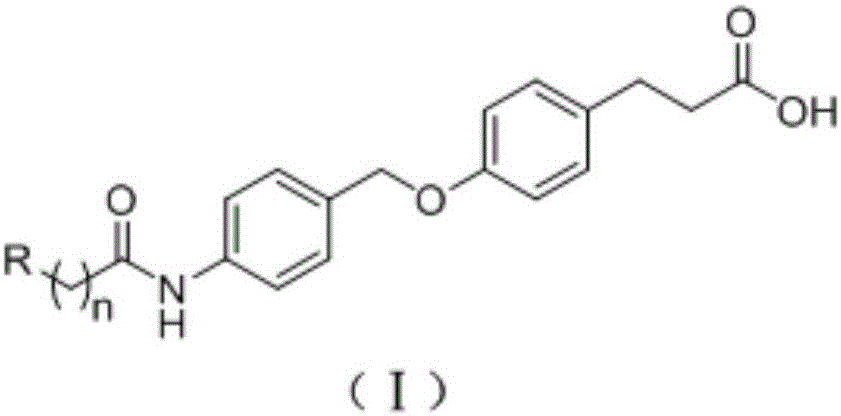
GPR120 (G-protein Coupled Receptor 120) small-molecule fluorescent probe and application of GPR120 small-molecule fluorescent probe
A fluorescent probe, small molecule technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
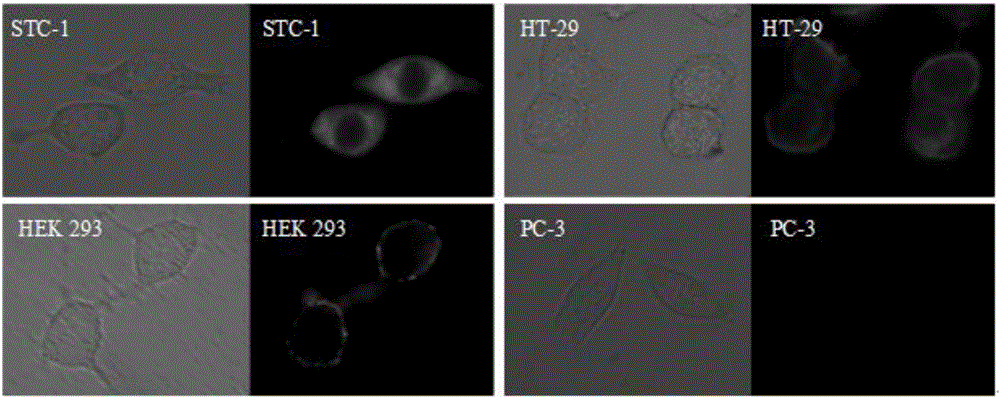
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of Naphthalimide Fluorophore Probes (L1-L3):
[0060]
[0061] The specific synthetic route is as follows:
[0062]
[0063] Preparation of Intermediate 1
[0064] In a 100 mL round-bottomed flask, add p-hydroxyphenylpropionic acid (1.5 g, 9.03 mmol) and anhydrous methanol (35 mL), stir to dissolve, add a catalytic amount of concentrated sulfuric acid (15 drops) dropwise, and stir at 60 ° C for 6 After 1 hour, it was lowered to room temperature, the reaction solution was spun out, water and dichloromethane were added for extraction, the organic layers were combined, washed with saturated brine, and anhydrous MgSO 4 It was dried overnight, filtered, concentrated and purified by column chromatography to obtain 1.54 g of a colorless and transparent oil with a yield of 95%. 1 H NMR (400MHz, DMSO): δ9.15(s, 1H), 6.99(d, J=8.4Hz, 2H), 6.65(d, J=8.5Hz, 2H), 3.57(s, 3H), 2.73( t, J=7.6Hz, 2H), 2.54 (t, J=7.6Hz, 2H); EI-MS: ([M] + ): 180.1.
[0...
Embodiment 2
[0090] Example 2: Preparation of coumarin-based fluorophore probes (L4-L7):
[0091]
[0092] The specific synthetic route is as follows:
[0093]
[0094] Preparation of Intermediate 7
[0095] In a 100mL round bottom flask, add 4-(diethylamino) salicylaldehyde (1.5g, 7.76mmol) and 40mL of absolute ethanol, then add diethyl malonate (1.24g, 7.76mmol), stir to make Dissolve morpholine (68 mg, 0.78 mmol) and acetic acid (20 μL) in 2 mL of absolute ethanol to obtain a mixed solution, then add the mixed solution to the above reaction solution, reflux and stir for 24 h, cool the reaction solution at ice temperature, and The reaction solution was spin-dried to obtain intermediate 7, which was directly used in the next step without purification.
[0096] Preparation of intermediate 8
[0097] Dissolve intermediate 7 (400mg, 1.38mmol) in a small amount of methanol solution, add 25mL of 2M NaOH solution, stir at room temperature for 24 hours, adjust the pH with 1M HCl to make t...
experiment example 1
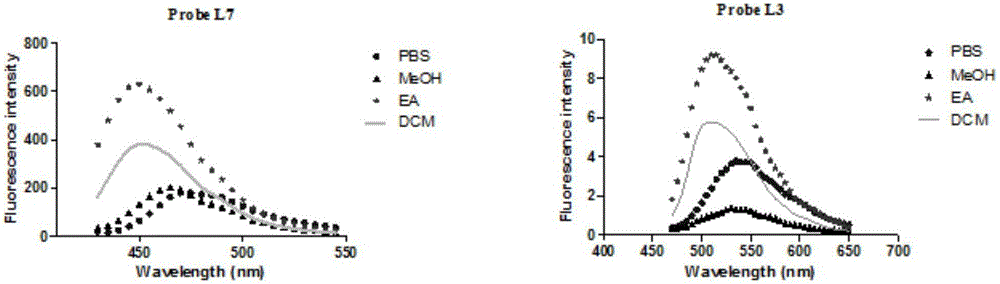
[0139] Experimental Example 1: Optical Activity Measurement
[0140] Table 1: Optical properties of probe molecules
[0141]
[0142]
[0143] Note: All the above optical properties were measured in Tris-HCl buffer pH=7.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com