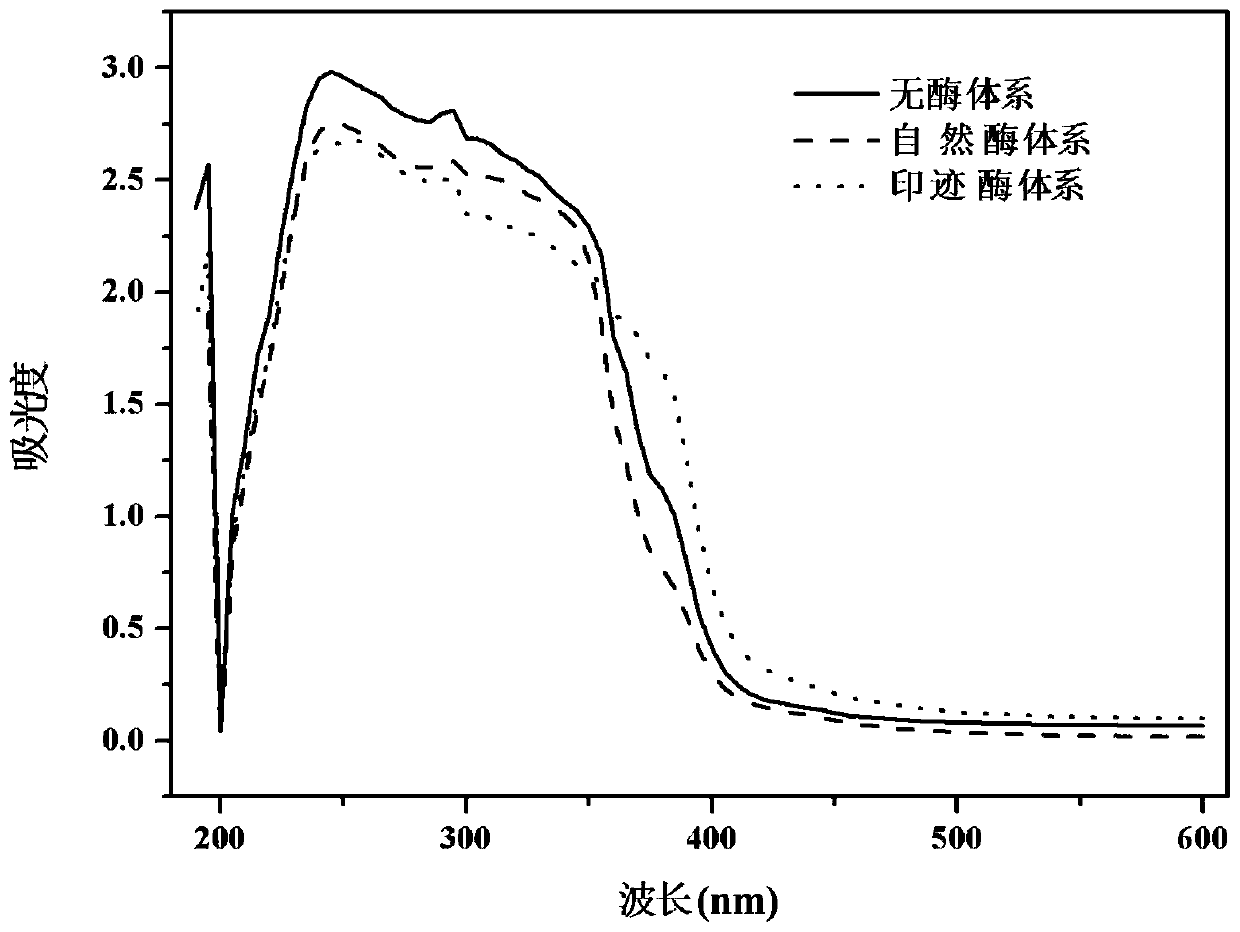
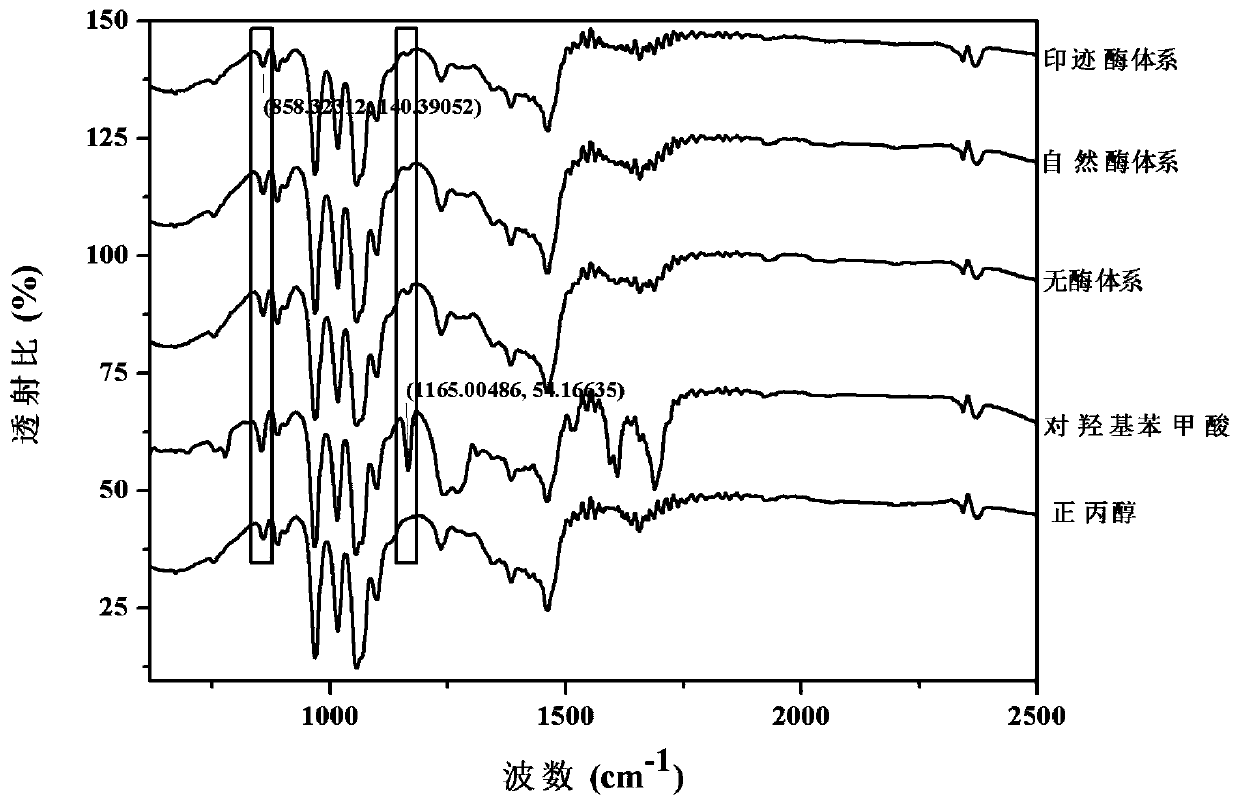
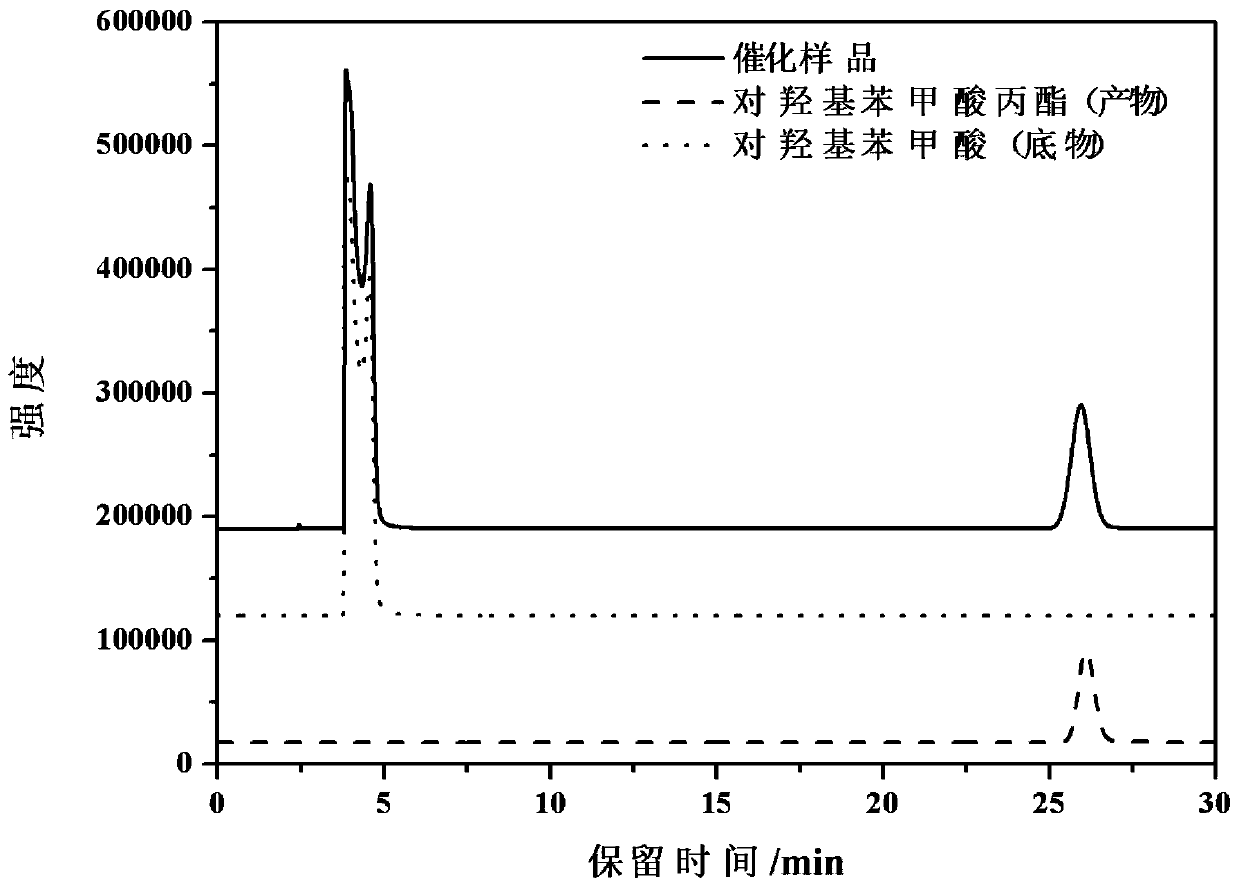
A kind of synthetic method of propyl p-hydroxybenzoate
A technology of propyl hydroxybenzoate and synthesis method, which is applied in the field of biocatalytic synthesis, can solve problems such as unreported research, and achieve the effects of low equipment requirements, low cost, and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0023] Experimental Example 1: Reaction Feasibility Study
[0024] 1. Preparation of bioimprinting enzymes
[0025] Add 1 mL of 50 mg / mL p-hydroxybenzoic acid (PHBA), 0.2 mL of 250 mg / mL tannase, 0.5 g of diatomaceous earth, and 4 mL of acetic acid buffer into a 50 mL Erlenmeyer flask and vortex for 3-5 minutes. The above mixture was placed at -20°C overnight, then freeze-dried at a low temperature to form a powder, and stored at 4°C for future use.
[0026] 2. Preparation of reaction system
[0027] 2.1. Enzyme-free system: Add 1 mL of n-propanol, 18 mL of n-hexane, 1 mL of 50 mg / mL PHBA, 0.3 mL of acetic acid buffer, and 0.5 g of diatomaceous earth into a 50 mL Erlenmeyer flask to prepare a reaction system.
[0028] 2.2. Natural enzyme system: Add 1mL of n-propanol, 0.2mL of 250mg / mL tannase, 0.5g of diatomaceous earth, and 4mL of acetic acid buffer into a 50mL Erlenmeyer flask and vortex for 3-5min. The above mixture was placed at -20°C overnight, freeze-dried at low tem...
experiment example 2
[0042] Experimental example 2: Determination of the reaction system
[0043] 1. Selection of reaction medium
[0044] Add 4 mL of n-propanol to five 50 mL Erlenmeyer flasks equipped with immobilized imprinted tannase in 1 of Experimental Example 1, and then add 15 mL of n-hexane, n-pentane, n-propanol, benzene and petroleum ether ( 30~60℃), 1mL acetic acid buffer solution, and prepare the catalytic system. Place the Erlenmeyer flask containing the reaction system in a micro-shaking incubator at 40°C and 200 rpm, and catalyze the reaction for 24 hours. The result is as Figure 4 As shown, when the reaction medium is n-propanol, the catalytic performance of the enzyme is the best, and the conversion rate of the substrate is the highest, reaching 4.23% (Table 2). Therefore, n-propanol was chosen as the reaction medium.
[0045] Table 2 The substrate conversion rate of different media
[0046]
[0047]
[0048] 2. Determination of the optimal content of n-propanol
[0...
Embodiment 1
[0053] Add 1 mL of 50 mg / mL PHBA, 0.2 mL of 250 mg / mL tannase, 0.5 g of diatomaceous earth, and 4 mL of acetic acid buffer into a 50 mL Erlenmeyer flask and vortex for 3-5 minutes. The above mixture was placed at -20°C overnight, then freeze-dried at a low temperature to form a powder, and stored at 4°C for future use. To the 50 mL Erlenmeyer flask containing the prepared bioimprinted tannase, add a mixed solution of n-propanol-acetic acid buffer with a total volume of 20 mL, wherein the volume percentage of n-propanol is 50%. Prepared as a catalytic system. Place the Erlenmeyer flask containing the reaction system in a micro-shaking incubator at 40°C and 200 rpm, and catalyze the reaction for 24 hours. The conversion rate of the final substrate was 26.47%, and the purity was 99.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com